Marco Cingolani e Benedetta Giordano
Steroid Hormones and Bipolar Disorder: neuroprotection vs. neurodegeneration.
Introduction
Bipolar disorder , also known as manic-depressive illness, is a severe psychiatric illness characterized by extreme shifts in mood from depression to mania, as well as fluctuations in energy and activity levels.
- Mania is the period of euphoria, racing thoughts, restlessness, energy and much talking. During this period, the patient is most likely to engage in risky behaviors, including risky sex (due to higher libido), because he has an over self-confidence and his judgment may be impaired.
- Depression is the opposite mood: the patient feels extremely sad, cries a lot, has a sense of being worthless, energy levels are low, there are a loss of pleasures, anxiety, guilt and insomnia.
In both episodes there may be psychosis, during which patients can not differentiate fantasy from reality; symptoms include delusions and hallucinations.

What is Bipolar Disorder? - Medical News Today, 2013
The clinical picture of BD involves disruption of behavior, circadian rhythms, neurophysiology of sleep, neuroendocrine and biochemical regulation within the brain.
These functions are mediated by a network of interconnected neurotransmitter pathways, distributed mainly in the Limbic system, which determines behavioral effects and has a neuroprotective action on the Central Nervous System.
| Physiological role | Bipolar Disorder |
| Noradrenergic system | Involved in fight or flight response | ↑ during mania
↓ during depression |
| Serotoninergic system | Counteracts dopamine, inhibiting mood regulation, appetite, sleep, pain and movements | Imbalance during mania |
| Dopaminergic system | Facilitates emotional behavior and movements, all functions linked to the Reward system | ↓ during depression |
| Gabaergic system | Inibitory role, ensures a lower neurons activation | Imbalance in mood disorders |
Although, what is not clearly known is how and why neuroprotection granted by some factors, first of all steroid hormones, is diminished or even lost in patients with bipolar disorders.
The underlying neurobiology of bipolar disorder - World Psychiatry, 2003
Bipolar disorder and steroid hormones
- Adrenal and gonad hormones
There is important evidence that patients with mood disorders show some differences between male and female in the onset, course and outcome of their disorders. Some studies showed that women have an increased risk for developing major depression, ipomania and mixed episodes compared to males.
In particular, these studies have suggested that women are more likely to develop or relapse this disorder during four main periods:
- Premenstrual
- Pregnancy
- Post-partum
- Peri-menopause
In fact, these phases of women's life are characterised by intense hormonal fluctuations, especially of the steroid hormones produced by the ovary and the adrenal cortex under the regulation of the hypotalamic-pituitary axis.
The two main hormones, estradiol and progesterone, induce a long-term action on neurons by activating a wide range of intracellular receptors that modulate transcription and protein synthesis. For this reason, the brain is one of the targets of steroids hormones.
It have been identified some estrogen's and progesterone's receptors in many Limbic areas (hypothalamus, hippocampus, amygdala and prefrontal cortex) implied in emotional regulation. Indeed it was found that higher levels of circulating estrogen enhance the risk of depressive episodes, so women are more vulnerable to mood shifts.
GABAergic neuroactive steroids: a new frontier in bipolar disorders? - Behavioral and brain functions, 2012
Ovarian hormones, through intracellular signaling systems, interact with other factors contributing to the pathophysiology of BD.
BDNF (brain-derived neurotrophic factor)
BDNF is a member of the neurotrophin family and is known to regulate neuronal maturation, differentiation, survival, neurotransmission, and synaptic plasticity. These effects in the central nervous system involve the activation of its membrane receptors TrkB and p75, which can activate a range of intracellular signaling cascades such as:
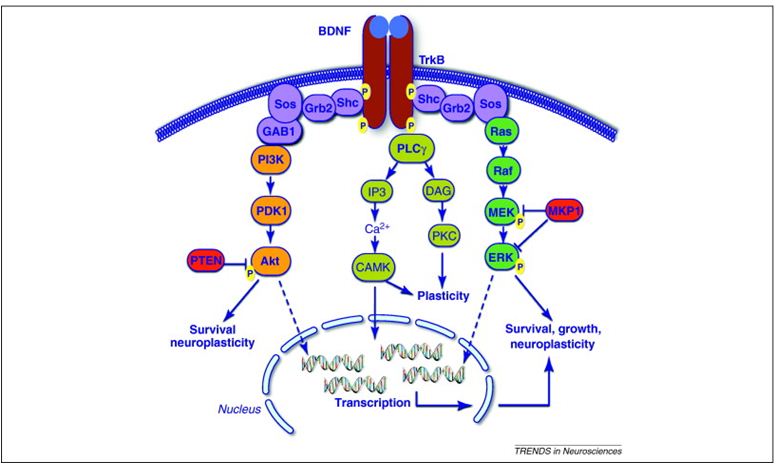
- PI3K-Akt;
- MAPK/ERK;
- PLC-γ;
- CAMK.
Through a study on ovariectomized rats (OVX) treated with estradiol it was found that BDNF mRNA was upregulated in their cerebral cortex and olfactory bulb, which indicates that estrogen can upregulate BDNF expression in forebrain neurons. Moreover, in vivo BDNF levels were found to be higher during the luteal compared to the follicular phase in fertile women.
Estradiol, upregulating BDNF, is associated with attenuation of neuronal loss and so has a neuroprotective effect.
However, several lines of evidence suggest that BD in humans is associated with decreased BDNF function: peripheral BDNF levels are decreased during manic and depressive episodes, and in subjects with history of multiple episodes.
So, BDNF may be an useful biomarker of mood state and progression of the disease.
ROS
An excessive generation of reactive oxygen species (ROS) can cause oxidative damages to lipids and protein and/or decrease enzymatic and non enzymatic defenses (OXIDATIVE STRESS).
They can lead to cellular apoptosis: the ROS increase causes an unbalance in positive charges in mitochondria that brings to an increase of Calcium influx through the Glu-channel, which activates some enzymes that promotes the apoptosis.
The excitotoxic and oxidative insults due to ROS can be diminished by estradiol and, to a lesser extent, by progesteron. Many of the neuroprotective effects of estrogen have been associated with stabilization of mitochondrial functions.
Estradiol increases mitochondrial energy efficiency and decreases the production of ROS, by the activation of his receptor ERα and the repression of the GLU receptor-mediated Ca ++ influx, avoiding the triggering of the apoptotic process.
Several studies have demonstrated that patient with BD have elevated circulating oxidative stress markers.
INFLAMMATION
Estradiol seems to reduce proinflammatory cytokines and chemokines in the central nervous system. Especially thanks to ERβ a specific signaling pathway is activated (CTBP-AP1) and ultimately represses genes that amplify inflammatory responses.
It has been revealed an increasing of IL levels in bipolar disorder, in particular of IL 2-4-6 during mania and only IL 6 during depression.
Sex hormones and biomarkers of neuroprotection and neurodegeneration: implications for female reproductive events in bipolar disorder - Wiley, 2013
PKC
PKC is an enzyme that modulates pre- and post-synaptic neurotransmitters release, regulating neuronal excitability and plasticity.
Some recent studies showed that estradiol activates PKC not only in other tissues, but also in cortical neurons: this PKC pathway seems to have a neuroprotective action, even if we don't know anything more yet.
PKC activity is seen to be increased in the prefrontal cortex following exposure to uncontrollable stress (STRESS -> NORADRENALINE -> α RECEPTORS -> PHOSPHATIDYL INOSITOL SIGNALLING PATHWAY). Due to the fact that the prefontal cortex mediates emotions, thoughts and actions, it is believed that an overactivity of PKC signalling is important for manic episodes of bipolar disorder, with an excessive neuronal activation.
Related to this, the PKC's substrate MARCKS (myristoylated alanine-rich C-kinase substrate) has been altered, too: normally, it is implied in the cross-linking between Actin's molecules, regulating cellular motility, secretion, transport... under the PKC control. PKC usually has a negative modulation: it phosophorilates MARCKS, detaching it from Actin. The increase of PKC's activity during manic episodes in BD, causes a decrease of MARCKS binded with Actin, which leads to an impairment in cognitive functioning.

For this reason, new antimanic drugs have been developed: PKC inhibitors. The chronic LITHIUM and VALPROATE administration resulted in reduced activity of several PKC isoforms in the frontal cortex and hippocampus.
In particular, Lithium acts on PLC-PIP2-DAG pathway inhibiting the enzyme Inositol-1-phosphatase, which catalyses the reaction IP->PIP; in this way, there is not enough level of PIP2, necessary for the PLC -> DAG -> PKC pathway. Therefore, PKC signalling is inhibited.

Litio - Pharmamedix
Valproate seems to act in a similar way on PKC, but its main role concerns the GABAergic system potentiation.
Moreover, novel therapeutic for acute mania are being studied: TAMOXIFEN, a synthetic anti-estrogen normally used in the treatment of breast cancer, was recently discovered as a PKC inhibitor. It's the only one selective inhibitor that crosses the blood-brain barrier. However, researchers are still working on it, even if it seems clear that tamoxifen has beneficial antimanic effects. It is important to emphasise that tamoxifen is also an anti-estrogen: so, it is possible that some of the antimanic effects are attributable to estrogen receptor antagonism.

Estrogen activates protein kinase C in neurons: role in neuroprotection, 2003
Protein Kinase C Inhibitors: Rationale for Use and Potential in the Treatment of Bipolar Disorder, 2009
- Neurosteroids
Besides adrenal and gonad hormones, it has been found the existence of neurosteroids directly synthesized in the brain (mainly in some glutammatergic neurons of the cortex, the hippocampus and the amydgala) from circulating steroid precursors. Currently neuroactive steroids are classified as pregnane neurosteroids (allopregnanolone and allotetrahydrodeoxycorticosterone or THDOC), androstane neurosteroids (androstanediol and etiocholanone) or sulfated neurosteroids (pregnenolone sulfate or PS and dehydroepiandrosterone sulfate or DHEAS).

Neurosteroids interact especially with neuronal membrane receptors and ion channels: the most important sites are the post-synaptic GABA A receptors:
- Allopregnanolone and THDOC are strong positive allosteric modulators of these receptors: their binding opens them and determine a chloride influx, which leads to a hyperpolarization. It potentiates inhibitory GABAergic transmission.


- Steroid sulfates are antagonists of these receptors and they have a negative modulatory action, which causes a reduction in channel opening.
The role of allopregnanolone and THDOC has been studied in mood disorders, it was speculated that these neurosteroids would act as endogenous mood stabilizers: indeed during depressive episodes the level of allopregnanolone is low, in order to avoid an excessive GABA A activity, while on the contrary during manic episodes its concentration is elevated.
By the way, in BD it has been discovered that neurosteroids are no longer an efficient way to stabilize mood: there is an evidence that their concentrations were lower in those who had shown manic compared with nonirritable patients.
This dysregulation has been discovered to be normalized through some drugs, first of all the FLUOXETINE: this drug, which usually works as serotonin reuptake inhibitor, stabilizes the level of neurosteroids in mood episodes. Generally it is administered in combination with OLANZAPINE, producing an increase in allopregnenolone's levels with an antimanic action. Fluoxetine triggers the enzyme 3β-hydroxysteroid dehydrogenase (3β-HSD), which catalyses the formation of allopregnenolone.
Fluoxetine 
GABAergic neuroactive steroids: a new frontier in bipolar disorders? - Behavioral and brain functions, 2012
Paroxetina e neurosteroidi, 2011
Conclusions
Bipolar disorder is clearly a complex psychiatric illness due to uncountable factors discovered day-by-day endlessly and not totally understood yet. By the way, what is evident is that a big part of this disease concerns a loss of neuroprotection, normally ensured by the interaction between steroid hormones and the complex CNS pathways. At this point, it appears important for researcher to examine new therapies based on these interactions.