Author : Luca Vespi
DESCRIPTION
Insulin Degludec is a long-acting basal insulin modified such that the amino acid residue
threonine in position B30 of human insulin has been omitted and the ε-amino group of lysine at position B29.
in position B29 has been coupled to hexadecanedioic acid via a glutamic acid spacer.


INDICATIONS
In patients with type 2 diabetes mellitus , insulin degludec can be administered alone, in combination with oral anti-diabetic products as well as in combination with bolus insulin. In type 1 diabetes mellitus , insulin degludec must be combined with short-/rapid-acting insulin to cover mealtime insulin requirements. Insulin degludec is to be dosed in accordance with the individual patient’s needs. It is recommended to optimise glycaemic control via dose adjustment based on fasting plasma glucose. As with all insulin products adjustment of dose may be necessary if patients undertake increased physical activity, change their usual diet or during concomitant illness.
Insulin degludec has been developed in two strengths as insulin degludec 100 U/ml and insulin degludec 200 U/ml, both being clear and colourless solutions containing the drug substance insulin degludec in a concentration of 600 nmol/ml and 1200 nmol/ml.
Insulin degludec 100 U/ml is intended to be marketed in two presentations, as a Penfill 3ml cartridge for use with durable pens and as a pre-filled disposable PDS290 pen-injector with a dose range of 1-80 U/injection, which can be dialled in 1 U increments
Insulin degludec 200 U/ml is intended for the market in a pre-filled disposable PDS290 pen-injector with a dose range of 2-160 U/injection, which can be dialled in 2 U increments.
European Medicines Agency,september 2012
MOLECULAR MECHANISM
Insulin degludec is active at a physiologic pH. The addition of hexadecanedioic acid to lysine at the B29 position allows to form soluble and stable multi-hexamers, resulting in a depot in the subcutaneous tissue after injection. The gradual separation of IDeg monomers from the multi-hexamers results in a slow and continuous delivery of IDeg from the subcutaneous injection site into the circulation, leading to the observed long pharmacokinetic and pharmacodynamic profiles. As a result of the changed pharmacokinetics with continuous release of IDeg monomers from the soluble multihexamer subcutaneous depot, IDeg has a longer duration of action than currently available basal insulin analogues such as insulin glargine (IGlar) and insulin detemir (IDet).
DEVELOPMENT
Evolution of insulin: from human to analog,2014 Oct
The development of insulin analogs has made improved treatment of type 2 diabetes possible.The flatter activity profiles of insulin glargine and insulin detemir translate into good clinical efficacy with a lower risk of hypoglycemia relative to neutral protamine Hagedorn insulin. Weight gain is consistently lower with insulin detemir than with neutral protamine Hagedorn insulin. Insulin degludec , licensed in Europe and Japan but not yet in the United States, has a mean half-life of 25.4 hours, a duration of action of >42 hours, and low variability.
EFFECTS
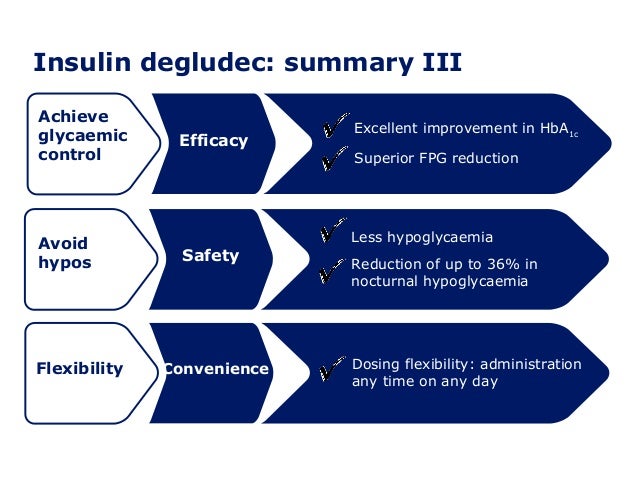
Insulin degludec has an onset of action of 30–90 minutes (similar to insulin glargine and insulin detemir). There is no peak in activity, due to the slow release into systemic circulation. The duration of action of insulin degludec is reported as being longer than 24 hours.
Basal-bolus treatment with insulin degludec was superior to long-acting insulin analogues detemir and glargine in reducing the rate of nocturnal hypoglycaemia. In comparison with other long-acting analogues, treatment with insulin degludec was safe and patients obtained similar metabolic control expressed by HbA1c and FPG levels with the added benefit of a reduced basal and total insulin dose.


CLINICAL TRIALS
Type 1 diabetes mellitus
Insulin degludec was studied as an alternative to insulin glargine as part of a basal-bolus regimen in the BEGIN Basal-Bolus Type 1 trial. 629 patients with type 1 diabetes were randomized in a 3:1 ratio to either insulin degludec (n=472) or insulin glargine (n=157) in addition to mealtime insulin aspart. Patients in the degludec treatment arm were switched from their basal insulin to insulin degludec in a 1:1 ratio, with a 20-30% dose reduction in patients receiving multiple basal doses per day. After 52 weeks, patients treated with insulin degludec produced a similar reduction in HbA1c (0.40% vs. 0.39%) meeting the criteria for noninferiority. Adverse events were similar in the two treatment arms; however, rates of nocturnal hypoglycemia (between midnight and 6am) were 27% lower in patients treated with insulin degludec (3.91 vs. 5.22%,p=0.024). The reduction in the incidence of hypoglycemia was seen as a therapeutic benefit, as hypoglycemia is often a dose limiting toxicity in insulin therapy.
Type 2 diabetes mellitus
In the BEGIN Basal-Bolus Type 2 trial, insulin degludec was studied as an alternative to insulin glargine in patients with type 2 diabetes mellitus. 995 patients were randomized to receive either insulin degludec (n=755) or insulin glargine (n=251), in addition to either mealtime insulin aspart, metformin , and/or pioglitazone. Patients in this trial had an average HbA1c of 8.3-8.4%, and 49-50% were on a regimen consisting of basal-bolus insulin + oral antidiabetic medications. After 52 weeks, insulin degludec was found to be noninferior to insulin glargine, providing a similar HbA1c lowering effect (-1.10 vs. -1.18%). Overall rates of hypoglycemia were significantly lower with insulin degludec (11.09 vs. 13.63%/yr, p=0.0359), including cases of nocturnal hypoglycemia (1.39 vs. 1.84%/yr, p=0.0399).
Wikipedia,2012 April
PHARMACODYNAMICS
Pharmacokinetic and pharmacodynamic responses of insulin degludec in African American, white, and Hispanic/Latino patients with type 2 diabetes mellitus , apr 2014 :
The purpose of this study was to investigate whether the pharmacokinetic and pharmacodynamic responses to IDeg at steady state vary according to patient race/ethnicity.
This randomized, single-center, double-blind, 2-period crossover trial investigated responses to IDeg in 59 patients with type 2 diabetes mellitus from 3 groups: African American, Hispanic/Latino, and white. Patients were allocated randomly to a sequence of 2 treatment periods, separated by a 7- to 21-day washout period, with once-daily IDeg or insulin detemir dosing for 6 days at a predefined fixed dose level (0.6 U/kg). Differences in pharmacokinetic and pharmacodynamic variables among groups were analyzed using an ANOVA with treatment period, an interaction between race/ethnicity, and treatment as fixed factors, subject as a random effect, and residual variance, depending on treatment.
RESULTS:
Total exposure to IDeg during one dosing interval at steady state (AUCIDeg,τ,SS) was similar among the racial/ethnic groups (ratio [95% CI]: African American vs white, 1.10 [0.91-1.31]; African American vs Hispanic/Latino, 1.13 [0.95-1.34]; and Hispanic/Latino vs white, 0.97 [0.82-1.16]). The total glucose-lowering effect of IDeg (AUCGIR,τ,SS) was also similar among the groups, with no statistically significant difference in pairwise comparisons (1940, 1735, and 2286 mg/kg in African American, white, and Hispanic/Latino patients, respectively). Steady state was reached in all groups after 2 to 3 days of dosing. In all groups, both exposure and glucose-lowering effect for IDeg were evenly distributed between the first and second 12 hours of the 24-hour dosing interval at steady state (mean AUCIDeg,0-12h,SS/AUCIDeg,τ,SS = 53%-54%; AUCGIR,0--12h,SS/AUCGIR,τ,SS = 47%-52%).
CONCLUSION:
The similar pharmacokinetic and pharmacodynamic responses to IDeg in 3 racial/ethnic groups of patients with type 2 diabetes mellitus suggest that the flat, stable, and ultralong pharmacokinetic and pharmacodynamic profiles of IDeg are preserved irrespective of race/ethnicity. Although insulin doses must be adjusted on an individual basis, similar pharmacokinetic and pharmacodynamic responses to IDeg are observed in patients with differing race/ethnicity.
Pharmacokinetic properties in subjects with hepatic impairment,2014 :
The ultra-long pharmacokinetic properties of insulin degludec were preserved in subjects with hepatic impairment and there were no statistically significant differences in absorption or clearance compared with subjects with normal hepatic function.
TOXICITY
The general toxicity of insulin degludec was assessed after s.c. single-dose administration in rats and dogs and after s.c. repeat-dose administration in rats and dogs for up to 52 and 26 weeks, respectively. In studies of 26 weeks duration or longer, recombinant human Neutral Protamine Hagedorn insulin (NPH insulin) was included as comparator to differentiate between effects considered related to pharmacological action of insulin and possible toxic effects of insulin degludec.
Dosing of insulin degludec to healthy normo-glycaemic animals lowered blood glucose to levels belowthe normal physiological concentration and thereby induced clinical signs of hypoglycaemia and hypoglycaemia-related mortality. These effects were dose-limiting factors in both species tested. In addition, the effect on blood glucose resulted in compensatory adaptive changes such as increased body weight gain and food consumption, various changes in clinical pathology, decreased liver weight and depletion of liver glycogen. The changes seen were similar in nature and magnitude to those induced by NPH insulin and showed recovery. The changes were considered related to pharmacological
effects of insulin and not unexpected toxic effects.
CARCINOGENICITY
Insulin degludec showed no carcinogenic potential in a 52-week toxicity study in Sprague Dawley rats upon complete histopathological evaluation of all animals. The female mammary gland was the focus of special attention and no treatment-related increase in incidences of hyperplasia, benign or malignant tumours was recorded in females dosed with insulin degludec. No treatment related changes in the female mammary gland cell proliferation were found using BrdU incorporation.
GENOTOXICITY
In accordance with the ICH S6 guideline, genotoxicity studies were not performed as insulin degludec is considered a biotechnology-derived product. Insulin degludec consists of desB30 human insulin, glutamate and 1,16-hexadecanedioic acid and none of the individual components are considered to possess a mutagenic potential. Glutamate is a commonly used food additive and mutagenicity has been investigated and found negative in Ames test and in vitro chromosomal aberration test.
Hexadecanedioic acid being a long-chain dicarboxylic fatty acid, and in general, fatty acids are not considered to possess a mutagenic potential.
EMA,2012 September
