DEFINITION
Sirtuins catalyse a unique deacetylation reaction in which NAD is consumed as a co-substrate. During the reaction process, the substrate protein is deacetylated and NAD is cleaved to nicotinamide and 2'-O-acetyl-ADP-ribose. NAD-dependent deacetylase activity is exhibited by all sirtuins except SIRT4, which is an ADP-ribosyl transferase that transfers an ADP-ribosyl group to the protein

THE GENE
| Database | Link | Link | Link |
| HGNC | "SIRT5": | "SIRT6": | "SIRT7": |
| Uniprot | "SIR5_HUMAN": | "SIR6_HUMAN": | "SIR7_HUMAN": |
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage
The Protein Aminoacids Percentage gives useful information on the local environment and the metabolic status of the cell (starvation, lack of essential AA, hypoxia)
Protein Aminoacids Percentage (Width 700 px)


| Database | Link | Link | Link |
| SIRT1 | Nucleus, cytoplasm | deacetylase |
| SIRT2 | Nucleus, cytoplasm | deacetylase |
| SIRT6 | Nucleus | Demyristoylase, depalmitoylase, ADP-ribosyl transferase and deacetylase |
| SIRT7 | nucleolar | deacetylase |


mitochondrial
- SIRT3 deacetylase
- SIRT4 ADP-ribosyl transferase
- SIRT5 Demalonylase, desuccinylase, and deacetylase

SIRT_Evolution
SYNTHESIS AND TURNOVER
mRNA synthesis
protein synthesis
post-translational modifications
degradation
CELLULAR FUNCTIONS
cellular localization,
biological function

The ways and means that fine tune Sirt1 activity, 2013
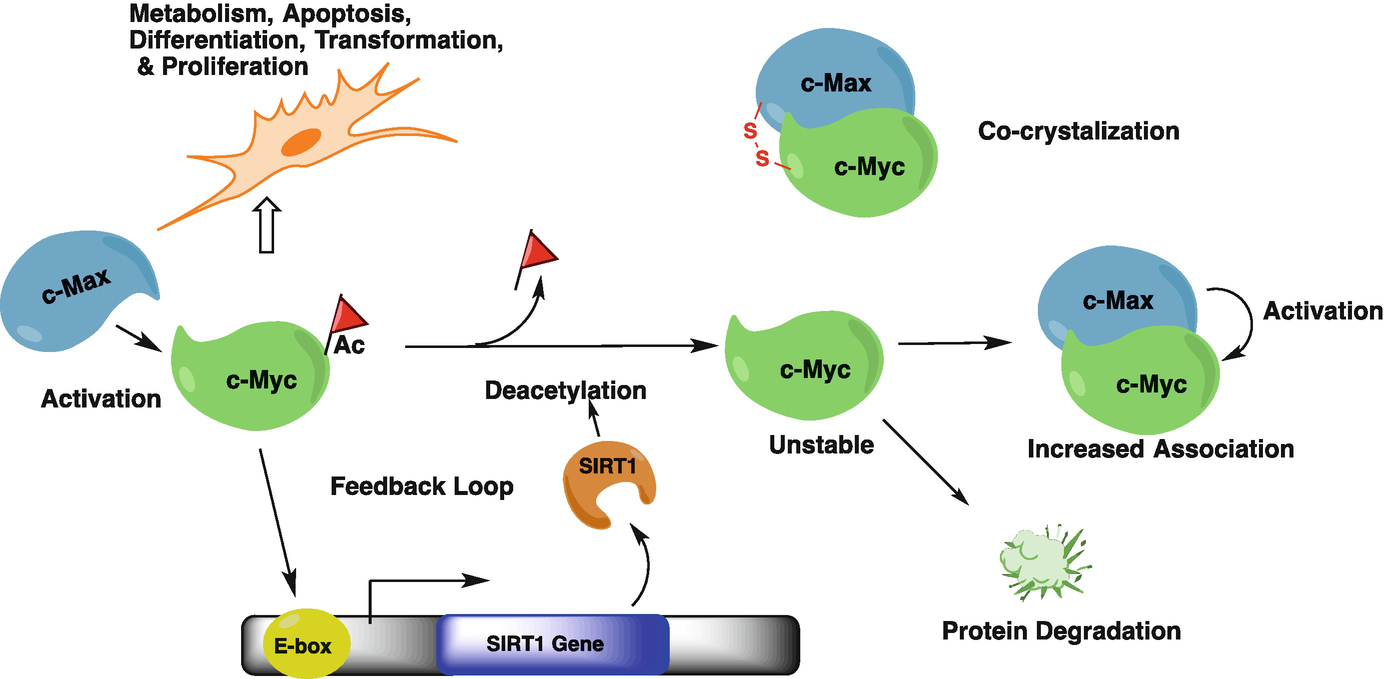
Role of SIRT1 in Epigenetics, 2019
SIRT1 mobilizes fat from white adipose tissue by blocking the activity of peroxisome proliferator-activated receptor-gamma (PPAR-gamma) through its interaction with nuclear receptor co-repressor (NCOR). b | SIRT1 represses the transcriptional activity of nuclear factor-kappaB (NF-kappaB) through deacetylation of the p65 subunit, and through interaction with transducin-like enhancer of split 1 (TLE1). c | The direct role of SIRT1 in insulin secretion from pancreatic beta-cells via the repression of UCP2 transcription is illustrated. Evidence exists for a role of SIRT1 in the regulation of insulin signalling pathways through deacetylation of the insulin receptor substrate 2 (IRS2) and repression of protein tyrosine phosphatase 1B (PTP1B). d | Cellular metabolic function controlled by mitochondrial number and function is modulated by SIRT1 through deacetylation of PPAR-gamma co-activator 1alpha (PGC1alpha). e | Cell survival under stress conditions is mediated through the interaction of SIRT1 with FOXO proteins. f | Werner syndrome (WRN) and Nijmegen breakage syndrome (NBS1) proteins are targets for SIRT1 and these interactions contribute to genomic stability.

The effects on inflammation, mitochondriogenesis and metabolism may be seen in more than one tissue. The broad actions of SIRT1 activation in these target tissues are illustrated.
Sir 2 and the Free [NAD+]/[NADH] Ratio
The levels of Sir2 and the [NAD+]/[NADH] ratio decrease as muscle cells start differentiating. Since the deacetylase activity of Sir2 is regulated by NAD+ availability, it is possible that the enzymatic activity of the remaining Sir2 would decline during differentiation. We directly tested this hypothesis by immunoprecipitating Sir2 from undifferentiated and differentiated muscle cells and measuring its deacetylase activity. Unfortunately, we were unsuccessful since the immunoprecipitated Sir2 was enzymatically inactive unless exogenous NAD+ was provided in the deacetylation reaction (data not shown). In analogous experiments, Sir2 immunoprecipitated from Drosophila cells was also found to be inactive (Barlow et al., 2001). This is likely due to the labile and transient association of cellular NAD+ with Sir2, which is not preserved during the immunoprecipitation procedure. To circumvent this technical obstacle, we experimentally increased the [NAD+]/[NADH] ratio by exposing cells to pyruvate. Under these conditions, we observed an inhibition of muscle gene expression and differentiation. Conversely, L-lactate, which reduces the [NAD+]/[NADH] ratio, stimulates muscle gene expression. While modification of the [NAD+]/[NADH] ratio is likely to affect several cellular processes, our experiments have established that Sir2 senses fluctuations of this ratio and affects muscle gene expression. Recently, it has been proposed that NAM is a negative regulator of Sir2 in vivo (Bitterman, K.J., Anderson, R.M., Cohen, H.Y., Latorre-Esteves, M. and Sinclair, D.A., 2002. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277, pp. 45099–45107. Full Text via CrossRef | View Record in Scopus | Cited By in Scopus (184)Bitterman et al., 2002). Since NAM is derived from NAD+ during the Sir2-mediated deacetylation reaction, the inference that changes in the free [NAD+]/[NADH] ratio could also influence the steady-state levels of NAM is currently being investigated. The [NAD+]/[NADH] ratio is subject to fluctuations imposed by the rate of glycolysis where glucose is metabolized to pyruvate and NAD+ is employed as a cofactor (Krebs and Veech, 1969). Acting as the primary site of insulin-mediated glucose uptake, skeletal muscle controls whole-body glucose availability in response to diet and exercise. Furthermore, the redox state of a cell is influenced by oxygen tension, which is modified during embryo development. The deacetylase activity of Sir2 may sense modifications of the [NAD+]/[NADH] ratio or NAM concentrations and respond by rapidly adjusting gene expression to accommodate the metabolic demands that occur in response to food intake, fasting, and exercise, as well as during embryonic and fetal development.
Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. 2003
1. FUNCTION: NAD-dependent deacetylase, which regulates processes such as apoptosis and muscle differentiation by deacetylating key proteins. Deacetylates 'Lys-382' of p53/TP53 and impairs its ability to induce proapoptotic program and modulate cell senescence. Deacetylates TAF1B and thereby represses rDNA transcription by the RNA polymerase I. Involved in HES1- and HEY2- mediated transcriptional repression. Inhibits skeletal muscle differentiation by deacetylating PCAF and MYOD1. May serve as a sensor of the cytosolic ratio of NAD/NADH, which is essential in skeletal muscle cell differentiation. Despite some ability to deacetylate histones in vitro, such activity is either weak or inexistent in vivo. In case of HIV-1 infection, interacts with and deacetylates the viral Tat protein.
2. CATALYTIC ACTIVITY: NAD + an acetylprotein = nicotinamide + O- acetyl-ADP-ribose + a protein.
3. COFACTOR: Binds 1 zinc ion per subunit (By similarity).
4. ENZYME REGULATION: Inhibited by nicotinamide. Activated by resveratrol (and 1,25 OH2 Vit.D3) (3,5,4'trihydroxy-trans-stilbene), butein (3,4,2',4' tetrahydroxychalcone), piceatannol (3,5,3',4'tetrahydroxy-trans stilbene), Isoliquiritigenin (4,2',4'trihydroxychalcone), fisetin (3,7,3',4'-tetrahydroxyflavone) and quercetin (3,5,7,3',4' pentahydroxyflavone).
5. SUBUNIT: Interacts with TAF1B. Found in a complex with PCAF and MYOD1 (By similarity). Interacts with MLLT7/FOXO4, HES1, HEY2, p53/TP53 and PML.
6. SUBCELLULAR LOCATION: Nucleus. Note=Recruited to the nuclear bodies via its interaction with PML.
7. TISSUE SPECIFICITY: Widely expressed.
8. MISCELLANEOUS: Red wine, which contains resveratrol, may participate in activation of sirtuin proteins, and may therefore participate in an extended lifespan as it has been observed in yeast.
9. SIMILARITY: Belongs to the sirtuin family.
10. SIMILARITY: Contains 1 deacetylase sirtuin-type domain.
Concurrent regulation of AMP-activated protein kinase and SIRT1 in mammalian cells. 2009
We examined in HepG2 cells whether glucose-induced changes in AMP-activated protein kinase (AMPK) activity could be mediated by SIRT1, an NAD-dependent histone/protein deacetylase that has been linked to the increase in longevity caused by caloric restriction. Incubation with 25 vs. 5mM glucose for 6h concurrently diminished the phosphorylation of AMPK (Thr 172) and ACC (Ser 79), increased lactate release, and decreased the abundance and activity of SIRT1.* In contrast, incubation with pyruvate (0.1 and 1mM) for 2h increased AMPK phosphorylation and SIRT1 abundance and activity. The putative SIRT1 activators resveratrol and quercetin also increased AMPK phosphorylation. None of the tested compounds (low or high glucose, pyruvate, and resveratrol) significantly altered the AMP/ATP ratio*. Collectively, these findings raise the possibility that glucose-induced changes in AMPK are linked to alterations in SIRT1 abundance and activity and possibly cellular redox state.
high NAD+, resveratrol and quercetin increase the abundance and activity of SIRT1 --> AMPK phosphorylation
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
SIR4 hypoxia
DIAGNOSTIC USE
THERAPEUTIC USE
Sirtuins — novel therapeutic targets to treat age-associated diseases 2008

high NAD+, resveratrol and quercetin increase the abundance and activity of SIRT1 --> AMPK phosphorylation
Sir2
SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. 2013
- The Sir2 family of enzymes or sirtuins are known as nicotinamide adenine dinucleotide (NAD)-dependent deacetylases and have been implicated in the regulation of transcription, genome stability, metabolism and lifespan. However, four of the seven mammalian sirtuins have very weak deacetylase activity in vitro. Here we show that human SIRT6 efficiently removes long-chain fatty acyl groups, such as myristoyl, from lysine residues. The crystal structure of SIRT6 reveals a large hydrophobic pocket that can accommodate long-chain fatty acyl groups. We demonstrate further that SIRT6 promotes the secretion of tumour necrosis factor-α (TNF-α) by removing the fatty acyl modification on K19 and K20 of TNF-α. Protein lysine fatty acylation has been known to occur in mammalian cells, but the function and regulatory mechanisms of this modification were unknown. Our data indicate that protein lysine fatty acylation is a novel mechanism that regulates protein secretion. The discovery of SIRT6 as an enzyme that controls protein lysine fatty acylation provides new opportunities to investigate the physiological function of a protein post-translational modification that has been little studied until now.
SIRT2‐dependent IDH1 deacetylation inhibits colorectal cancer and liver metastases, 2020

SIRT2 overexpression significantly inhibits CRC cell proliferation, migration, and invasion. IDH1 acetylation is modulated in response to intracellular metabolite concentration and regulates cellular redox hemostasis. Moreover, IDH1 acetylation reversely regulates HIF1α‐dependent SRC transcription which in turn controls CRC progression
Regulation of G6PD acetylation by SIRT2 and KAT9 modulates NADPH homeostasis and cell survival during oxidative stress, 2014
- Abstract
Glucose-6-phosphate dehydrogenase (G6PD) is a key enzyme in the pentose phosphate pathway (PPP) and plays an essential role in the oxidative stress response by producing NADPH, the main intracellular reductant. G6PD deficiency is the most common human enzyme defect, affecting more than 400 million people worldwide. Here, we show that G6PD is negatively regulated by acetylation on lysine 403 (K403), an evolutionarily conserved residue. The K403 acetylated G6PD is incapable of forming active dimers and displays a complete loss of activity. Knockdown of G6PD sensitizes cells to oxidative stress, and re-expression of wild-type G6PD, but not the K403 acetylation mimetic mutant, rescues cells from oxidative injury. Moreover, we show that cells sense extracellular oxidative stimuli to decrease G6PD acetylation in a SIRT2-dependent manner. The SIRT2-mediated deacetylation and activation of G6PD stimulates PPP to supply cytosolic NADPH to counteract oxidative damage and protect mouse erythrocytes. We also identified KAT9/ELP3 as a potential acetyltransferase of G6PD. Our study uncovers a previously unknown mechanism by which acetylation negatively regulates G6PD activity to maintain cellular NADPH homeostasis during oxidative stress.


The major difference involves the ratio Asp/Asn very high in SIR2
Role of SIRT1 in Epigenetics, 2019 Book
Role+of+SIRT1+in+Epigenetics+Wang
Role+of+SIRT1+in+Epigenetics
SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function.2012
- Mice treated with a moderate dose of resveratrol showed increased mitochondrial biogenesis and function, AMPK activation, and increased NAD levels in skeletal muscle, whereas SIRT1 knockouts displayed none of these benefits. A mouse ov …
SIRT1 Resveratrol on Mitochondrial Function