DESCRIPTION
Tacrolimus (also FK-506) is an immunosuppressive drug that is mainly used after allogeneic organ transplant to reduce the activity of the patient's immune system and so lower the risk of organ rejection. It is also used in a topical preparation in the treatment of atopic dermatitis (eczema), severe refractory uveitis after bone marrow transplants, exacerbations of minimal change disease, and the skin condition vitiligo.
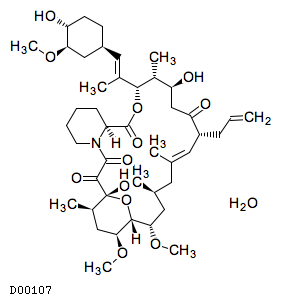
Tacrolimus was discovered in 1984 when a strain of Streptomyces tsukubaensis was found to produce the potent immunosuppressive agent, designated by the code number FK-506; it was among the first macrolide immunosuppressant discovered, preceded by the discovery of rapamycin. In vitro immune systems, it was showed that mixed lymphocyte reaction, cytotoxic T cell generation, the production of T cell-derived soluble mediators such as interleukin 2 (IL-2), interleukin 3 (IL-3) and gamma-interferon (IFN-γ) and the expression of the IL-2 receptor were suppressed by this agent. The IC50 values of FK-506 and cyclosporine (CS) in all tests were approximately 0.1 nM and 10 nM, respectively. Therefore, the novel agent, FK-506 suppressed in vitro immune systems at about hundred times lower concentration than CS.
FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. 1987
It was first approved by the Food and Drug Administration (FDA) in 1994 for use in liver transplantation; this has been extended to include kidney, heart, small bowel, pancreas, lung, trachea, skin, cornea, bone marrow, and limb transplants.
CLASSIFICATION
1. Immunosuppressive agents
2. Antirheumatics (DMARDs- disease-modifying antirheumatic drugs-)
3. Type I polyketide structures
INDICATIONS
• Immunosuppression following transplantation: It has similar immunosuppressive properties to cyclosporine, but is much more potent. A study to evaluate the beneficial and harmful effects of immunosuppression to cyclosporine versus tacrolimus for liver transplanted patients showed that tacrolimus is superior to cyclosporine in improving patient survival, graft survival, and in preventing acute rejection after liver transplantation, but increases post-transplant diabetes. Tacrolimus is normally prescribed as part of a post-transplant cocktail including steroids, mycophenolate and IL-2 receptor inhibitors.
Cyclosporin versus tacrolimus for liver transplanted patients.2006
• Dermatological use: As an ointment Protopic, tacrolimus is used in the treatment of eczema, particularly atopic dermatitis. It suppresses inflammation in a similar way to steroids, and is equally as effective as a mid-potency steroid. Tacrolimus 0.1% ointment was found to be of similar efficacy to class I/II and class III topical corticosteroids. With the exception that tacrolimus ointment caused more skin burning than comparator treatments, no significant differences with regards to side-effects and withdrawals were found. While one study reported greater improvements in tacrolimus-treated adult patients compared with topical steroids, the second reported greater improvements in pediatric patients treated with steroids compared with tacrolimus ointment. Pimecrolimus and tacrolimus are calcineurin inhibitors that are recommended as second-line treatment for persons with moderate to severe atopic dermatitis and who are at risk of atrophy from topical corticosteroids.
A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. 2011
Atopic dermatitis: an overview. 2012
In 2004 a study showed its effectiveness to treat segmental vitiligo in children, especially in areas on the face and neck. Also double-blind studies show that tacrolimus 0.1% ointment combined with excimer laser is superior to placebo, especially for UV resistant areas, such as bony prominences of the extremities. When used alone, tacrolimus 0.1% ointment is almost as effective as clobetasol propionate 0.05% ointment. Other studies suggest it can also be effective for facial lesions. Double blind studies show that Pimecrolimus 1% cream combined with narrow band UVB is superior to placebo, especially for facial lesions.
Efficacy of topical calcineurin inhibitors in vitiligo. 2013
Tacrolimus ointment promotes repigmentation of vitiligo in children: a review of 57 cases. 2004
• Antirheumatics: Disease-modifying Antirheumatic Drugs (DMARDs) is a category of otherwise unrelated drugs defined by their use in rheumatoid arthritis to slow down disease progression. The term is often used in contrast to non-steroidal anti-inflammatory drug (which refers to agents that treat the inflammation but not the underlying cause) and steroids (which blunt the immune response but are insufficient to slow down the progression of the disease). Although their use was first propagated in rheumatoid arthritis, the term has come to pertain to many other diseases, such as Crohn's disease, lupus erythematosus (SLE), immune thrombocytopenic purpura (ITP), myasthenia gravis and various others. Many of these are autoimmune disorders, but others, such as ulcerative colitis, are probably not. A clinical trial suggests that tacrolimus may be effective for short-term clinical improvement in patients with refractory ulcerative colitis. However, these results should be interpreted with caution due to the small number of patients enrolled in the trial and other study limitations.
Tacrolimus (FK506) for induction of remission in refractory ulcerative colitis. 2008
• Ocular Diseases: Systemic and topical tacrolimus have been investigated as treatments for ocular surface disorders that may have an immune-based inflammatory components. Tacrolimus has shown efficacy in corneal graft rejection, inflammatory conjunctival and corneal diseases, uveitis, and graft-versus-host disease. Uveitis treatment involves topical corticosteroids along with cycloplegic-mydriatics. Particularly severe cases may require systemic corticosteroids and immunosuppressive drugs. In patients refractory to traditional treatment, the use of 0.1% topical ophthalmic FK- 506 (tacrolimus) ointment has been occasionally reported.
Tacrolimus in the treatment of ocular diseases. 2011
Childhood chronic anterior uveitis associated with vernal keratoconjunctivitis (VKC): successful treatment with topical tacrolimus. Case series. 2011
• Minimal Change Disease: Recently, it has been proved the efficacy of low-dose, long-term tacrolimus for inducing and sustaining remission in children with steroid resistant and steroid-dependent nephrotic syndrome.
Treatment of childhood nephrotic syndrome with long-term, low-dose tacrolimus. 2013
PHARMACOKINETICS
Tacrolimus is available as an intravenous formulation, a capsule for oral use and an ointment for topic use. Currently, most of the pharmacokinetic data available for tacrolimus are based on an enzyme-linked immunosorbent assay method, which does not distinguish tacrolimus from its metabolites. The drug has low absorptivity, low blood and plasma concentrations.
• Absorption: the rate of absorption of tacrolimus is variable: in adult patients, it is generally rapidly absorbed following oral administration (the time to reach maximum concentration is 1 to 2 hours), but in some patients absorption is slow or even delayed. Because of presystemic elimination, the oral bioavailability is low (around 20%, less after eating food rich in fat) but may vary between 4 and 89%. Absorption of tacrolimus after topical application is dependent on the barrier function of the skin. Absorption through the intact epidermis is very low and eczematic skin a little higher. In comparison to systemic tacrolimus used for prevention and treatment of rejection after organ transplantation, the bioavailability of topical tacrolimus in patients with atopic dermatitis is between 3 and 4%.
• Distribution: tacrolimus is highly bound to erythrocytes. Its binding to plasma proteins (in particular albumin and alpha 1-acid glycoprotein) varies between 72 and 98% depending on the methodology used. Because of the extensive partitioning of tacrolimus into erythrocytes, its apparent volume of distribution (Vd) based on blood concentrations is much lower (1.0 to 1.5 L/kg) compared with values based on plasma concentrations (about 30 L/kg).
• Metabolism: tacrolimus is completely metabolised prior to elimination. It is metabolised in the liver by cytochrome P450 (CYP) 3A4 to at least 10 metabolites, some of which retain significant activity.
• Elimination: biliary excretion is the route of elimination of the tacrolimus metabolites, which is mostly faecal. Systemic plasma clearance is very high (0.6 to 5.4 L/h/kg), whereas blood clearance is much lower (0.03 to 0.09 L/h/kg). The terminal elimination half-life (t1/2beta) of tacrolimus is approximately 12 hours (with a range of 3.5 to 40.5 hours). Its elimination is decreased in the presence of liver impairment and in the presence of several drugs.
Various factors contribute to the large intra- and interindividual variability in the pharmacokinetics of tacrolimus. Only limited information is available in paediatric patients. The rate and extent of tacrolimus absorption after oral administration do not seem to be altered. The Vd based on blood concentrations (2.6 L/kg) is approximately twice the adult value. Blood clearance of tacrolimus is also approximately twice as high in paediatric (0.14 L/h/kg) compared with adult (0.06 L/h/kg) patients. Consequently, t1/2beta does not appear modified in children, but oral doses need to be generally 2-fold higher than corresponding adult doses to reach similar blood concentrations.
Clinical pharmacokinetics of tacrolimus. 1995
Comparative clinical pharmacokinetics of tacrolimus in paediatric and adult patients. 2001
MOLECULAR MECHANISM
• In T-cells, activation of the T-cell receptor normally increases intracellular calcium, which acts via calmodulin to activate calcineurin. Calcineurin then dephosphorylates the transcription factor NF-AT (nuclear factor of activated T-cells), which moves to the nucleus of the T-cell and increases the activity of genes coding for IL-2 and related cytokines. Cyclosporin A (CsA), pimecrolimus and tacrolimus block T cell activation by inhibiting the phosphatase calcineurin and preventing translocation from the cytoplasm to the nucleus of the transcription factor Nuclear Factor of Activated T cells (NFAT). NFAT compose a family of transcription factors that are turned on during T cell activation. In detail, Tacrolimus reduces peptidyl-prolyl isomerase activity by binding to the immunophilin FKBP12 (FK506 binding protein) creating a new complex. This FKBP12-FK506 complex interacts with and inhibits calcineurin thus inhibiting both T-lymphocyte signal transduction and IL-2 transcription.

Modulation of NFAT-5, an outlying member of the NFAT family, in human keratinocytes and skin. 2009
Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. 1991
• Cyclosporin A (CsA) and tacrolimus are also utilized for the treatment of inflammatory skin diseases such as psoriasis. The therapeutic effects are thought to be mediated via their immunosuppressive action on infiltrating lymphocytes in skin lesions. Calcineurin and NFAT 1 have been shown to be functionally active in cultured human keratocytes, as well as other NFAT family members such as NFAT-2, -3 and -5. Moreover, the expression of NFAT-2 in normal skin, non-lesional and lesional psoriasis suggests a role for NFAT-2 in keratocytes proliferation. As with NFAT 1, differentiation-promoting agents that increase intracellular calcium concentration induced nuclear translocation of NFAT-2 in cultured keratocytes but with different kinetics. NFAT-2 may play an important role in regulation of keratocytes proliferation and differentiation at a different stage. Inhibition of this pathway in human epidermal keratocytes many account, in part for the therapeutic effects of CsA and tacrolimus in skin disorders such as psoriasis. Thus, calcineurin/NFAT is functionally active not only in T cells, but in skin cells. The same process can be seen for NFAT-3.
Expression, localisation and functional activation of NFAT-2 in normal human skin, psoriasis, and cultured keratocytes. 2009
A preliminary examination of the role of NFAT 3 in human skin, cultured keratocytes and dermal fibroblasts. 2010
• Cyclosporin A (CsA) and tacrolimus (FK506), independent of immunophilin binding, can activate profibrogenic transforming growth factor β TGFβ/Smad signaling cascades in rat renal mesangial cells (MC), inducing renal fibrosis. Via a cyclophilin-independent pathway, via increased ROS production, they can activate TGFβreceptor-triggered signaling pathways by activating rapid release of latent ECM-bound TGFβ. Thereby, the Smad-2-dependent pathway is convergently activated through p38 and TGFβ-triggered signaling. The association of Smad-2 with Smad-4 (Smad-2/Smad-4) gives rise to an active Smad transcription factor complex that specifically binds to a cognate SBE present in the promoters of CTGF and TIMP-1 genes.

Molecular mechanisms of TGF beta receptor-triggered signaling cascades rapidly induced by the calcineurin inhibitors cyclosporin A and FK506. 2008
• They also activate the extracellular-signaling regulated kinase ERK, a member of the mitogen activated protein kinase (MAPK), and induce a rapid and transient increase in ERK phosphorylation. ERK activation is mediated via HB-EGF-induced EGF receptor (EGFR) tyrosine kinase activation. CsA and FK506 via a rapid generation of mitochondrial ROS can induce proteolytic activity of metalloproteinases, like ADAM17, which cause shedding of the membrane-bound growth factor HB-EGF. HB-EGF itself transactivates the EGF-receptor and activates signaling events downstream of the EGFR including activation of ERK1/2. The increase in ERK1/2 activity presumably will lead to transcriptional induction mainly of those genes which mediate proliferative responses in renal mesangial cells.

Cyclosporin A and tacrolimus induce renal Erk1/2 pathway via ROS-induced and metalloproteinase-dependent EGF-receptor signaling. 2012
• Tacrolimus inhibits the expressions of Ang-1, Tie-2, and VEGF by blocking the activations of the IL-1β-mediated JNK and p38 MAPK pathways in human FLS (fibroblast-like synoviocytes) . This suggests that tacrolimus contributes to the suppression of angiogenesis in the pathogenesis of RA.
Tacrolimus (FK506) inhibits interleukin-1β-induced angiopoietin-1, Tie-2 receptor, and vascular endothelial growth factor through down-regulation of JNK and p38 pathway in human rheumatoid fibroblast-like synoviocytes. 2012
PHARMACOGENOMICS
Tacrolimus is metabolic substrate for cytochrome P450 (CYP) 3A enzymes--in particular, CYP3A4 and CYP3A5--and is transported out of cells via P-glycoprotein (ABCB1). Several single nucleotide polymorphisms SNPs have been identified in the genes encoding for CYP3A4, CYP3A5 and P-glycoprotein, including CYP3A4 -392A>G (rs2740574), CYP3A5 6986A>G (rs776746), ABCB1 3435C>T (rs1045642), ABCB1 1236C>T (rs1128503) and ABCB1 2677G>T/A (rs2032582). Influence of the CYP3A4 -392A>G SNP on the pharmacokinetics of either ciclosporin or tacrolimus appears limited. Variability in CYP3A4 expression due to environmental factors is likely to be more important than patient genotype. The CYP3A5 6986A>G SNP has a well-established influence on the pharmacokinetics of tacrolimus. Several studies in kidney, heart and liver transplant recipients have reported an approximate halving of tacrolimus dose-adjusted trough concentrations and doubling of tacrolimus dose requirements in heterozygous or homozygous carriers of a CYP3A5*1 wild-type allele compared with homozygous carriers of a CYP3A5*3 variant allele. Carriers of a CYP3A5*1 allele take a longer time to reach target blood tacrolimus concentrations. Influence of ABCB1 3435C>T, 1236C>T and 2677G>T/A SNPs on the pharmacokinetics of ciclosporin and tacrolimus remains uncertain. Genetic linkage between the three variant genotypes suggests that the pharmacokinetic effects are complex and not related to any one ABCB1 SNP. It is likely that these polymorphisms exert a small but combined effect, which is additive to the effects of the CYP3A5 6986A>G SNP. In liver transplant patients, recipient and donor liver genotypes may act together in determining overall drug disposition, hence the importance of assessing both. Further haplotype analyses are likely to be useful. It is not yet clear whether pharmacogenetic profiling of calcineurin inhibitors will be a useful clinical tool for personalizing immunosuppressant therapy.
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. 2010
SIDE EFFECTS
• From oral and intravenous administration
Side effects can be severe and include infection, cardiac damage, hypertension, blurred vision, liver and kidney problems (tacrolimus nephrotoxicity), hyperkalemia, hypomagnesemia, hyperglycemia, diabetes mellitus, itching, lung damage (see also Tacrolimus-induced lung injury in a rheumatoid arthritis patient with interstitial pneumonitis. 2008) and various neuropsychiatric problems such as loss of appetite, insomnia, Posterior reversible encephalopathy syndrome, confusion, weakness, depression, cramps, neuropathy, seizures, tremors, and catatonia. In addition it may potentially increase the severity of existing fungal or infectious conditions such as herpes zoster or polyoma viral infections.
The chronic nephrotoxicity of these drugs is the Achilles' heel of current immunosuppressive regimens. Its development is related to both reversible alterations and irreversible damage to all compartments of the kidneys, including glomeruli, arterioles, and tubulo-interstitium. The main question--whether nephrotoxicity is secondary to the actions of cyclosporine and tacrolimus on the calcineurin-NFAT pathway--remains largely unanswered. Local exposure to cyclosporine or tacrolimus could be more important than systemic exposure. Finally, other local susceptibility factors for calcineurin inhibitor nephrotoxicity are variability in P-glycoprotein and CYP3A4/5 expression or activity, older kidney age, salt depletion, the use of nonsteroidal anti-inflammatory drugs, and genetic polymorphisms in genes like TGF-beta and ACE.
Calcineurin inhibitor nephrotoxicity. 2009
Carcinogenesis and mutagenesis In people receiving immunosuppressants to reduce transplant graft rejection, an increase risk of malignancy is a recognised complication. The most common cancers are non-Hodgkin's lymphoma and skin cancers. The risk appears to be related to the intensity and duration of treatment. For many years, the increased risk of skin cancer have been attributed to the immunosuppression due to decreased immunological tumor monitoring, which leads to reduced control and elimination of near tumor formation and thus more cancer, including the inhibition of antigen-presenting cells and decreased proliferation and function of T cells. Immunosuppression also promotes viral infections, which can affect the development of certain forms of cancer. Many believe that infection with the virus may play a role in the development of cutaneous squamous cell carcinoma by organ transplantation, but this is not clarified. Calcineurin inhibitors have been shown to promote the development of cancer also via non-immune mechanisms. Immunosuppressive therapy is a risk factor for the increased incidence and metastatic progression of malignancies in organ graft recipients. Transforming growth factor TGF-beta1 has been associated with tumor invasion and metastasis. Tacrolimus has a dose-dependent effect on tumor progression and TGF-beta1 expression, and tacrolimus-induced TGF-beta1 overexpression may be a pathogenetic mechanism in tumor progression. It is also shown that calcineurin inhibitors delay apoptosis and inhibit the repair of DNA damage in keratinocytes after UV exposure. Mouse studies have shown the necessary role of intact p53 protein, which has protective effect against the development of squamous cell carcinoma. This may explain why calcineurin inhibitors increases the risk of skin cancer, and suggest that this effect is related to the disappearance of the tumor protective role of p53 protein.
Immunosuppressive drugs and the development of skin cancer after organ transplantation. 2012
Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. 2003
• From topical use
The most common adverse events associated with the use of Protopic, especially if used over a wide area, include a burning or itching sensation on the initial applications. Less common are flu-like symptoms, headache and cough and burning eyes. There may be also an increased risk of certain skin infections.
Cancer risks
Tacrolimus and a related drug for eczema (pimecrolimus) were suspected of carrying a cancer risk, though the matter is still a subject of controversy. The FDA issued a health warning in March 2005 for the drug, based on animal models and a small number of patients. Until further human studies yield more conclusive results, the FDA recommends that users be advised of the potential risks. See the Public Health Advisory for Elidel and Protopic. 03/10/2005.
In 2009, during a follow-up period of 4 years in clinical studies, no increased risk of infections or cancer was associated with long-term use of tacrolimus ointment. Only short-term adverse events were detected. They included increased burning and stinging of the skin, and a temporary increase in skin infections. No signs of immunosuppression were observed after 1 - 4 years of intermittent treatment with tacrolimus ointment. Despite these findings, many physicians remain concerned about possible long-term malignancies associated with long-term treatment with a topical calcineurin inhibitor. Long term safety of tacrolimus ointment in atopic dermatitis. 2009
In 2011, during a study, cases of lymphoma or cutaneous cancer have been observed following use of topical calcineurin inhibitors (TCIs), but it is unclear whether TCI use increases cancer risk. . However, there are only sparse data on specific malignancies among TCI-treated patients. The short duration of typical TCI exposure hinders conclusions about longer exposure. There is insufficient evidence in the epidemiological literature to infer whether TCIs do or do not cause malignancy.
Evaluation of cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. 2011
TOXICITY
• Pregnancy after solid organ transplantation, although considered high risk for maternal, fetal, and neonatal complications, has been quite successful. Tacrolimus pharmacokinetic changes during pregnancy: there are multiple factors that can increase the fraction of unbound tacrolimus, including but not limited to low albumin concentration and low red blood cell count. The clinical titration of dosage to maintain whole blood tacrolimus trough concentrations in the usual therapeutic range can lead to elevated unbound concentrations and possibly toxicity in pregnant women with anemia and hypoalbuminemia. Tacrolimus crosses the placenta with in utero exposure being approximately 71% of maternal blood concentrations. The lower fetal blood concentrations are likely due to active efflux transport of tacrolimus from the fetus toward the mother by placental P-glycoprotein. To date, tacrolimus has not been linked to congenital malformations but can cause reversible nephrotoxicity and hyperkalemia in the newborn. In contrast, very small amounts of tacrolimus are excreted in the breast milk and are unlikely to elicit adverse effects in the nursing infant.
Interpreting Tacrolimus Concentrations During Pregnancy and Postpartum. 2012
• There is a case of a living-donor liver transplant recipient experiencing a considerable increase in the trough blood concentration of tacrolimus after concomitant ingestion of grapefruit juice (250 mL) 4 times for 3 days. The trough blood concentrations of tacrolimus were not changed during or immediate after the repeated intake of grapefruit juice. However, almost 1 week after the final ingestion, the blood concentration of tacrolimus markedly increased to as much as 47.4 ng/mL from 4.7 ng/mL before the ingestion, resulting in a profound reduction of calcineurin phosphatase activity in peripheral blood mononuclear cells. Furthermore, headache and nausea, but not nephrotoxicity or hyperglycemia, took place throughout the period of the elevated blood concentrations. Grapefruit juice may have a clinically significant effect on the pharmacokinetics and pharmacodynamics of tacrolimus. It is recommended to avoid the consumption of grapefruit juice in transplant recipients treated with tacrolimus.
Delayed effect of grapefruit juice on pharmacokinetics and pharmacodynamics of tacrolimus in a living-donor liver transplant recipient. 2006
RESISTANCE
• During a study about Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR, they found that Tacrolimus rapidly and effectively induced remission of SRNS in Chinese adults with disease refractory to treatment with intravenous cyclophosphamide. However, treatment may be less effective in patients with FSGS (focal segmental glomerulosclerosis).
Tacrolimus therapy in adults with steroid- and cyclophosphamide-resistant nephrotic syndrome and normal or mildly reduced GFR. 2009