Definition
Intrinsically photosensitive retinal ganglion cells are a rare sub-population of ganglion cells (1-3%) whose primary role is to signal light for unconscious visual reflexes, such as pupillary constriction and regulating a number of daily behavioral and physiological rhythms, collectively called circadian rhythms.
They are a third type of mammalian photoreceptor that differs greatly from rods and cones.
Indeed they utilize a different photopigment, are much less sensitive to light, and have far less spatial resolution.
These photoreceptors are ganglion cells, and thus, have the unique ability to communicate directly with the brain. This latter process, which adjusts circadian rhythms to the light/dark cycle of an animal's environment, is known as photoentrainment.

The unique ability of ipRGCs to respond to light is due to their exclusive expression of the photopigment melanopsin.
Originally cloned from frog dermal melanophores, the melanopsin gene (OPN4) has orthologs in many mammalian species, including mice, monkeys, and humans (Provencio, Rodriguez et al. 2000).
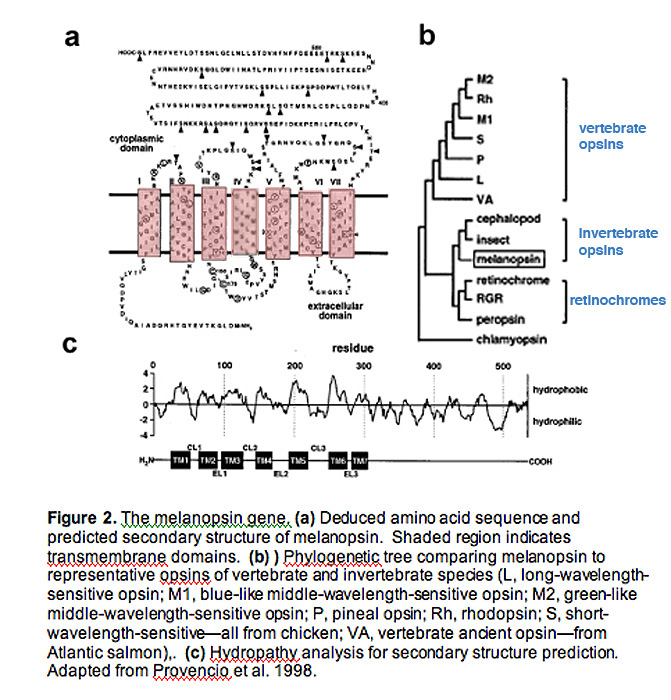
Hydrophobicity analysis of melanospin's amino-acid sequence predicts a 7-transmembrane structure, common to all G-protein coupled receptors (Fig. 2a, 2c) (Provencio, Jiang et al. 1998).
Interestingly, melanopsin shares more homology with invertebrate rhabdomeric opsins (r-opsins) than with ciliary opsins of vertebrate species (c-opsins), suggesting that melanopsin may signal light through a different mechanism than that used in vertebrate rods and cones (Fig. 2b) (Provencio, Jiang et al. 1998)
The rodless-coneless (rd/rd cl) mouse proved valuable in characterizing the photopigment of pRGCs.
The known photopigments of animals consist of an opsin protein linked to a chromophore which is a specific form of vitamin A called 11-cis retinal. All opsin/vitamin A-based photopigments have a characteristic absorption profile that allows these photopigments to be identified on the basis of their spectral responses to light or action spectra. The process of generating an action spectrum involves the protracted task of measuring a series of full doseâÃÂÃÂresponse curves for a range of monochromatic stimuli. The first full action spectrum to define the nature of the non-rod, non-cone photopigment studied pupil constriction in rd/rd cl mice. The results revealed a previously uncharacterized, opsin/vitamin A-based photopigment with peak sensitivity at 479 nm, which was tentatively named OP479 (opsin photopigment ÃÂûmax 479 nm) (R.J. Lucas et al. 2001) Subsequently, action spectra from mice to human have been deduced for a range of responses including the direct recording from pRGCs in rats (D.M. Berson et al. 2002) and in macaques (D.M. Dacey et al. 2005) clear consensus has emerged from these studies demonstrating the existence of a common single novel opsin photopigment with a ÃÂûmax of around 480 nm.

Central Projection
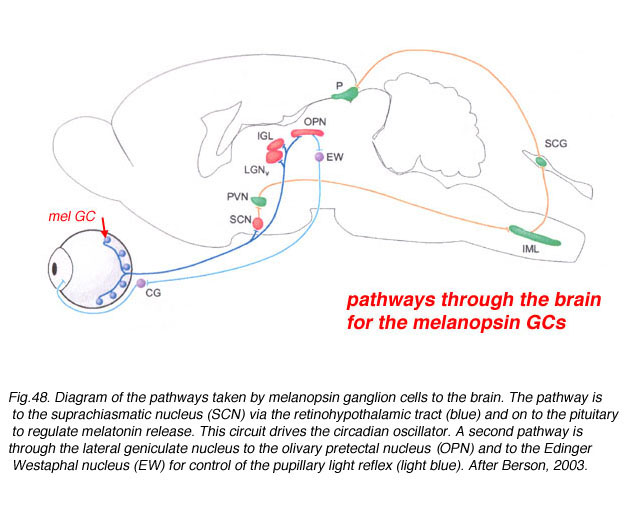
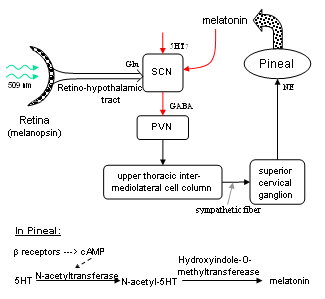
The axons of the ipRGCs belonging to the retinohypothalamic tract project directly to the suprachiasmatic nuclei via the optic nerve and the optic chiasm. The suprachiasmatic nuclei receive and interpret information on environmental light, dark and day length, important in the entrainment of the 'body clock'. A polysynaptic circuit that originates in the SCN and photically regulates melatonin by the pineal gland (P) through its sympathetic innervation.
Behavioral aspects of ipRGC function
Daily rhythms in mammalian physiology and behavior, collectively called circadian rhythms, are controlled by a tiny cluster of cells in the suprachiasmatic nuclei (SCN) of the ventral hypothalamus.
SCN neurons posses a unique molecular clock that allows them to autonomously regulate activity patterns in near 24-hour rhythms, which ultimately leads to daily changes in animal behaviors such as sleep/wake cycles. However, the clocks are not tuned perfectly to 24 hours, and in order to adjust to changes in the animals environmental light/dark phases, the SCN must be entrained (or reset). Light is by far the most potent entrainment cue, and ipRGCs are the primary cells that carry this daily signal. Melanopsin knockout animals have relatively normal circadian rhythms, and do not display any overt dysfunction in their ability to entrain to light (Fig. 15) (Panda, Sato et al. 2002; Ruby, Brennan et al. 2002).
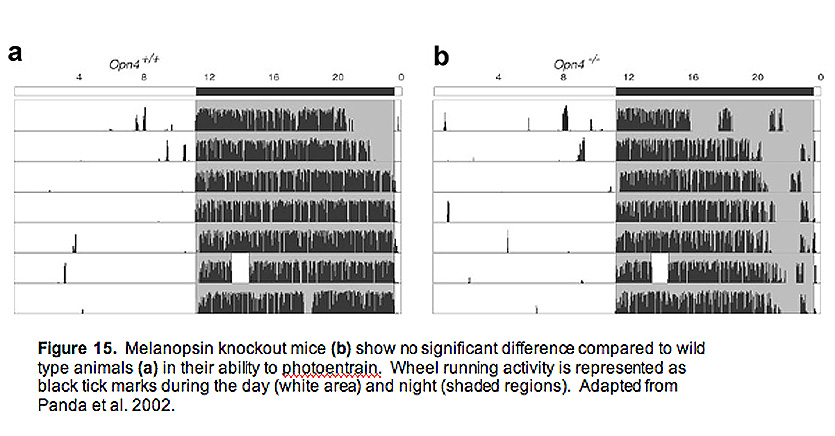
Measured both behaviorally and using cFos staining (a measure of activity in neurons) in the SCN, these animals show no significant difference to wild type animals (Panda, Sato et al. 2002; Ruby, Brennan et al. 2002). However, they display a significantly attenuated phase shifting response (Fig. 16) to brief flashes of light, indicating a crucial role for ipRGCs and melanopsin signaling (Panda, Sato et al. 2002; Ruby, Brennan et al. 2002). The insignificant change in photoentrainment of melanopsin knockout animals is most likely explained by the rod and cone input to ipRGCs, adding a redundant source of photic input to the SCN. This idea was tested directly by doing behavioral analysis on melanopsin knockout mice that lack rods and cones (Hattar, Lucas et al. 2003; Panda, Provencio et al. 2003). In addition, Samer Hattar and colleagues developed an ingenious mouse line where genes encoding key enzymes in rod and cone signaling, as well as the melanopsin gene, were all deleted, leaving the cellular architecture of the retina completely intact, but devoid of all phototransduciton capabilities (Hattar, Lucas et al. 2003). Much like the melanopsin knockout mice without rods and cones, these "triple knockout" mice could neither phase-shift their circadian rhythms to brief pulses of light, nor could they photoentrain to light/dark cycles (Hattar, Lucas et al. 2003).
 Melanopsin and inner retinal photoreception.
Melanopsin and inner retinal photoreception.
 Melanopsin: an exciting photopigment.
Melanopsin: an exciting photopigment.
 Intrinsically photosensitive retinal ganglion cells.
Intrinsically photosensitive retinal ganglion cells.
Links to pathology
Blindness
 Melanopsin is required for non-image-forming photic responses in blind mice.
Melanopsin is required for non-image-forming photic responses in blind mice.
 Non-visual photoreception: sensing light without sight.
Non-visual photoreception: sensing light without sight.
 Suppression of melatonin secretion in some blind patients by exposure to bright light
Suppression of melatonin secretion in some blind patients by exposure to bright light
Seasonal Affective Disorder (SAD)
 A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder.
A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder.


SAD, Eating disorders
 Seasonal mood patterns in eating disorders.
Seasonal mood patterns in eating disorders.
 Carbohydrate craving. Relationship between carbohydrate intake and disorders of mood.
Carbohydrate craving. Relationship between carbohydrate intake and disorders of mood.
SAD and Hormons
Vitamin D and the occurrence of depression: causal association or circumstantial evidence? 2009
 Vitamin D and mood disorders among women: an integrative review.
Vitamin D and mood disorders among women: an integrative review.
 Plasma leptin in men and women with seasonal affective disorder and in healthy matched controls.
Plasma leptin in men and women with seasonal affective disorder and in healthy matched controls.
 Rate of oxygen consumption in seasonal and non-seasonal depression.
Rate of oxygen consumption in seasonal and non-seasonal depression.

Links to therapy
 Illuminating rationale and uses for light therapy.
Illuminating rationale and uses for light therapy.
 Blue in the face
Blue in the face
 The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder.
The Can-SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder.
"!http://flipper.diff.org/static/files/912/pubmed-22.gif! Blue-blocking IOLs Decrease Photoreception without Providing Significant Photoprotect