DEFINITION
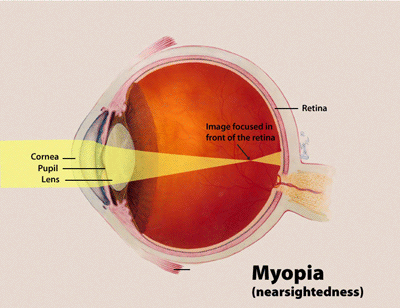
Myopia (Greek: μυωπία, muōpia, "nearsightedness" (AmE), "shortsightedness" (BrE)) is a refractive defect of the eye in which collimated light produces image focus in front of the retina under conditions of accommodation. In simpler terms, myopia is a condition of the eye where the light that comes in does not directly focus on the retina but in front of it. This causes the image that one sees when looking at a distant object to be out of focus but in focus when looking at a close object.
EPIDEMIOLOGY
The global prevalence of refractive errors has been estimated from 800 million to 2.3 billion.
The incidence of myopia within sampled population often varies with age, country, sex, race, ethnicity, occupation, environment, and other factors.
PATHOGENESIS
Theoretically myopia can occur for 3 reasons:
1. The eye is longer than usual; this is termed axial myopia. This is the commonest form of myopia.
2. The cornea is more curved than usual and so is stronger. The light is focused too far forward, in front of the retina.
3. The lens of the eye becomes stronger, as in early cataract formation. The stronger lens focuses the light too far forward.
PATIENTS RISK FACTORS
Vascular
Myopia affects well over 30% of adult humans globally. However, the underlying physiological mechanism is little understood. This study tested the hypothesis that ocular growth and refractive compensation to optical defocus can be controlled by manipulation of potassium and chloride ion-driven transretinal fluid movements to the choroid.
The homeostatic control of eye growth functions to keep images sharply focused on the retina. Therefore, if the eye length increases more slowly than does the focal length, the focal plane will be behind the retina, creating hyperopic defocus on the retina. The same occurs if one puts a negative lens over the eye. To regain sharp focus, the retina needs to be displaced backward to where the image is. This is done in two ways: the eye is lengthened by increasing the rate of growth or of remodeling of the sclera at the posterior pole of the eye (Gentle and McBrien 1999 and Nickla et al. 1997), and the retina is pulled back within the eye by the thinning of the choroid, the vascular layer between the retina and sclera (Wallman et al. 1995 and Wildsoet and Wallman 1995); once distant images are again focused on the retina (emmetropia), both the rate of ocular elongation and the choroid thickness return to normal.
In chicks, studies have shown that the increased thickness results mostly through the expansion of the lacunae in the stroma of the choroid (Junghans et al., 1999). The lacunae are membrane-bound spaces that are part of the lymphatic drainage of the eye (De Stefano and Mugnaini 1997; Junghans et al. 1999 and Wallman et al. 1995). The mechanism of the expansion is not known. One possibility is that the synthesis of osmotically active molecules draws water into the lacunae. This is supported by the findings that glycosaminoglycan production in the choroid increases during expansion (Nickla et al. 1997 and Wallman et al. 1995), and that, if glycosaminoglycan production is reduced by xyloside treatment, choroidal thickness is also reduced (Rada et al., 2002). Alternatively, choroidal thickness might be regulated via control of fluid transport into and out of the choroid. In support of this possibility, changes in choroidal blood flow precede changes in choroid thickness following form deprivation or recovery (Fitzgerald et al., 2002), and ultrastructural correlates of increased fluid transport into the lymphatic vessels occur during recovery from form-deprivation myopia (Junghans et al., 1999).
Chicks were raised with + /-10D or zero power optical defocus rendering the focal plane of the eye in front of, behind, or at the level of the retinal photoreceptors respectively. Intravitreal injections of barium chloride, a non-specific inhibitor of potassium channels in the retina and RPE or bumetanide, a selective inhibitor of the sodium-potassium-chloride cotransporter were made, targeting fluid control mechanisms. Comparison of refractive compensation to 5mM Ba2+ and 10−5 M bumetanide compared with control saline injected eyes shows significant change for both positive and negative lens defocus for Ba2 + but significant change only for negative lens defocus with bumetanide






Vitreous chamber depths showed a main effect for drug conditions with less depth change in response to defocus shown for Ba2+ relative to Saline, while bumetanide injected eyes showed a trend to increased depth without a significant interaction with applied defocus. The results indicate that both K channels and the NKCC cotransporter play a role in refractive compensation with NKCC blockade showing far more specificity for negative, compared with positive, lens defocus. Probable sites of action relevant to refractive control include the apical retinal pigment epithelium membrane and the photoreceptor/ON bipolar synapse. The similarities between the biometric effects of NKCC inhibition and biometric reports of the blockade of the retinal ON response, suggest a possible common mechanism. The selective inhibition of refractive compensation to negative lens in chick by loop diuretics such as bumetanide suggests that these drugs may be effective in the therapeutic management of human myopia.
Potassium Channel and NKCC Cotransporter Involvement in Ocular Refractive Control Mechanisms
Control of Eye Growth by Visual Signals
Genetics
Population, family, and twin studies also have provided evidence for a genetic component to high myopia. According to a nationwide survey in Taiwan in 2000, girls have a higher prevalence and more severe myopia progression than boys. Several other epidemiological studies have also shown similar results. In addition, the female sex was reported to be independently associated with faster myopia progression. Because of sex differences, a hormonal hypothesis has been postulated by a review article examining myopia in opposite-sex twins. In animal models, sex differences were also demonstrated in chick eye growth and experimental myopia. It has also been shown that myopia progresses quickly in childhood, especially around puberty. These studies have demonstrated that sex may be a modulating factor affecting myopia and have suggested that the differential expression of sex hormones may be as well. Knowledge about sex differences in the progression of myopia and about the pathophysiology underlying those differences may enhance the accuracy and effectiveness of clinical assessment and treatment of myopia.
Methods
A campus-based sample of 283 cases (145 males and 138 females) with high myopia and 280 controls (144 males and 136 females) with low myopia or emmetropia was studied. Estradiol, progesterone, and testosterone levels were determined using enzyme-linked immunosorbent assay kits. We genotyped six SNPs within five steroidogenesis enzyme genes (17 alpha-hydroxylase/17,20 lyase [CYP17A1], 3 beta-hydroxysteroid dehydrogenase [HSD3B1], 17 beta-hydroxysteroid dehydrogenase 1 [HSD17B1], steroid-5-alpha-reductase, alpha polypeptide 2 [SRD5A2], and aromatase [CYP19A1]) using polymerase chain reaction–restriction fragment length polymorphism methods. Student’s t-tests, χ2 tests, logistic regression, multifactor dimensionality reduction (MDR) methods, and ANOVA were used to determine significance.
Results
An MDR analysis corroborated the synergistic genotype association and demonstrated that synergistic interaction between rs6203 (HSD3B1), rs10046 (CYP19A1), and sex might confer susceptibility to high myopia (p=0.019). In both male and female subjects, levels of testosterone were significantly higher in cases than in controls; in male subjects, the levels of estradiol were significantly higher and those of progesterone were significantly lower in cases (all p-values <0.001). The rs605059 (HSD17B1), with sex-gene interaction, showed association with estradiol levels in males (p=0.035) and testosterone levels in females (p=0.027).



Polymorphisms in steroidogenesis genes, sex steroid levels, and high myopia in the Taiwanese population
Hormonal
A diet rich in sugar and refined carbohydrate leads to higher insulin levels
The higher blood insulin level stimulates the liver to produce more IGF1, leading higher blood IGF1 levels. The IGF reaches the eye where it stimulates growth of the eyeball.
Methods
Chicks were treated with either positive or negative spectacle lenses and intravitreally injected with saline or different amounts of insulin. Refraction, axial length, and corneal curvature were measured. Effects of insulin on vitreal glucose concentration, on retinal ZENK and glucagon mRNA levels, and on the number of ZENK-immunoreactive glucagon amacrine cells were studied.
Results
Insulin injections (0.3 nmol) caused only a small myopic shift in control chicks. When positive lenses were worn, insulin injections (0.3; 0.03 nmol) not only blocked hyperopia but rather induced high amounts of axial myopia. Insulin also enhanced myopia that was induced by negative lenses. Axial elongation was mostly due to an increase in anterior chamber depth and a thickening of the crystalline lens. Insulin temporarily reduced vitreal glucose levels. Insulin increased retinal ZENK mRNA levels, whereas the number of ZENK-immunoreactive glucagon amacrine cells was reduced, a finding that is typically linked to the development of myopia.
Conclusions
Given that insulin is used in therapy for human metabolic disorders and has been proposed to treat corneal epithelial disease, its powerful myopiagenic effect, which is mostly due to its effects on the optics of the anterior segment of the eye, merits further investigation. Evidence for a potential role of glucagon during eye growth regulation in chicks.
It seems likely that amacrine cells contribute to the visual regulation of ocular growth and that glucagon may act as a stop signal for eye growth. The purpose of the present study was to accumulate further evidence for a role of glucagon in the visual control of eye growth. Chicks were treated with plus and minus lenses after injection of different amounts of the glucagon antagonist des-His1-Glu1-glucagon-amide or the agonist Lys17,18,Glu21-glucagon, respectively. Refractive development and eye growth were recorded by automated infrared photorefraction and A-scan ultrasound, respectively. The glucagon antagonist inhibited hyperopia development, albeit only in a narrow concentration range, and at most by 50%, but not myopia development. In contrast, the agonist inhibited myopia development in a dose-dependent fashion.

Insulin Acts as a Powerful Stimulator of Axial Myopia in Chicks
Evidence for a potential role of glucagon during eye growth regulation in chicks
Relative axial myopia in Egr-1 (ZENK) knockout mice
TISSUE SPECIFIC RISK FACTORS
Physiopathological (due to tissue function and activity)
Many experts now believe that the prevalence of myopia is due to some aspect of modern civilization perhaps learning to read and write at an early age—that interferes with the normal feedback control of vision on eye development, leading to abnormal elongation of the eyeball. A corollary of this hypothesis is that if children (or, more likely, their parents) wanted to improve their vision, they might be able to do so by practicing far vision to counterbalance the near work “overload.” (from D. Purves, G. J. Augustine, D. Fitzpatrick, W. C. Hall, A. LaMantia, J. O. McNamara, L. E. White, Neuroscience)
There is epidemiological evidence accumulating over decades that visual factors
might contribute to myopia in humans. The evidence is of three types. First, there are epidemiological studies in many countries showing an association between the educational level attained and the prevalence of myopia (e.g., Goldschmidt 1968 and Sperduto et al. 1983), ranging from 3% for unskilled laborers to 30% for those with university educations. Second, a high proportion of young adults who do intensive professional studies (medical, law, engineering, or pilot school) become myopic over the few years of study (e.g., Kinge et al. 2000 and Zadnik and Mutti 1987). Third, cultures in which people lead outdoor lives have little myopia (Morgan and Rose, 2004), but when compulsory education and the other attributes of modern Western culture were introduced to Inuit or American Indian villages, there was a 4-fold increase in the incidence of myopia within one generation (Bear, 1991), although it is difficult to dissociate the visual changes from dietary and other changes (Cordain et al., 2002). The thrust of these findings is that education is associated with an increased prevalence of myopia. The risk factor most discussed as the intervening variable is reading, because the nearness of the page presents the eye with hyperopic defocus.
Eye growth during normal childhood development coordinates with progressive changes in the optical power of the cornea and lens to maintain image focus on the plane of the retina. Observations after unilateral visual deprivation have indicated that retinal image quality influences postnatal growth. Deprivation of form vision in juvenile monkeys, chicks, or humans disrupts normal regulation and leads to excessive eye size; distant images now focus in front of the retina, causing a myopic refractive error. This link of visual quality to eye size implicates the nervous system in growth control. Moreover, recent observations hint that such control is largely local. (i) Form-deprivation myopia in both monkeys and chicks takes place even after optic nerve transection interrupts the direct pathway from retina to brain. (ii) Application of a partial occluder in chicks to restrict vision either in the nasal or temporal visual
field induces excessive eye growth only along the corresponding ocular dimension. For example, occlusion of the nasal visual field causes excessive growth of the temporal part of the globe.
The growth of the eyeball is strongly influenced by focused light falling on the retina. This phenomenon was first described in 1977 by Torsten Wiesel and Elio Raviola at Harvard Medical School, who studied monkeys reared with their lids sutured, a procedure that deprives the eye of focused retinal images. They found that animals growing to maturity under these conditions show an elongation of the eyeball. The effect of focused light deprivation appears to be a local one, since the abnormal growth of the eye occurs in experimental animals even if the optic nerve is cut. Indeed, if only a portion of the retinal surface is deprived of focused light, then only that region of the eyeball grows abnormally.
Although the mechanism of lightmediated control of eye growth is not fully understood.
As previously reported, unilateral visual deprivation by lid suture, translucent goggle, or transparent goggle resulted in excessive eye growth in both axial and equatorial dimensions. All three types of visual deprivation also reduced retinal concentrations of the catecholamine dopamine and its metabolite 3,4-dihydroxyphenylacetic acid (DOPAC), as measured in light-adapted birds at intervals during a 4-week observation period. Retinal concentrations of dopamine and DOPAC normally vary in accordance with the state of light/dark adaptation. Visual deprivation by translucent goggles for 2 weeks lessened the usual light-associated rise.


Effect of visual deprivation on ocular growth. Newborn White Leghorn chicks underwent unilateral visual deprivation by lid suture, translucent goggle, or transparent goggle. Unilateral visual deprivation results in excessive eye growth in both axial and equatorial dimensions (mean SEM; n = 5-13 birds in each group). Student's t statistics were used to compare paired differences
between deprived versus nondeprived eyes. N.S., not significant. *, P ' 0.001; **, P ' 0.01.
Retinal dopamine and form-deprivation myopia
THERAPY
Fluid movements across the RPE to the choroidal blood supply are controlled by the ionic channels, cotransporters and symporters of the apical and basal membranes of the RPE. In vitro eye cup studies demonstrate that numerous ionic species (K+, Na+, Ca2+, H+, Cl−, HCO3−, as well as lactate) affect subretinal space fluid dynamics. However, due to the fact that to date, only K, Na and Cl abundance have been experimentally associated with myopia, and given that Crewther's hypothesis related myopia to the activity of the inwardly rectifying K channels and the sodium potassium 2-chloride symporter (NKCC1) on the apical membrane of the RPE, we have chose to focus this study on potassium and the way in which potassium channels and cation-chloride transporters, particularly the sodium potassium 2-chloride symporter (NKCC1) on the apical membrane of the RPE, are intimately involved in the growth response to defocus.
NKCC1 belongs to the cation-chloride cotransporter family, which mediates the coupled movement of Na, K and two Cl ions in strict ratio across the plasma membrane of cells. NKCC1 transport is electroneutral, with the driving force for ion influx being in part supplied by the inward Na+ gradient and maintained by Na/K-ATPase. These channels, symporters and exchangers, driven by alterations in K concentration, modulate fluid transfer by controlling the absorption and secretion of chlorid, and hence water transport, via the cotransporters themselves and in concert with aquaporin channels.
Sulfamoybenzoic acid loop diuretics such as bumetanide can compete with Cl− for the second chloride binding site and thus inhibit NKCC1 function .
Cation-chloride transporters are found not only on epithelial cells, but also across the retina. Vardi et al used antibodies to mGluR6 to label the metabotropic glutamate receptor at the photoreceptor ON-bipolar synapse, and showed that antibodies to NKCC1 but not KCC2 (potassium chloride symporter receptor protein) co-localize with the glutamate receptor. Similarly, anti-calbindin, that selectively labels an OFF-bipolar subclass, co-localizes with KCC2 but not NKCC1 immunoreactivity at the flat synapses of the photoreceptor OFF-bipolar interface.
Thus, bumetanide, which is a selective blocker of NKCC1 may also alter retinal function by blocking the cation-chloride uptake at the ON-bipolar dendrites (and horizontal cells).
Given the likelihood that myopia induced by lens defocus shares many of the same mechanisms and ultrastructural changes as form deprivation, it is probable that inhibiting potassium movements in such retinae would interfere with defocus induced refractive and growth changes, especially as apical potassium and basal chloride appear to be strongly linked in the RPE.
The RPE contains many different potassium channels. Early Ussing chamber experiments demonstrated the presence of weak inwardly rectifying potassium channels (Kir), with more recent research delineating the likely presence of Kir7.1 and Kir4.1 subfamilies. Despite their inwardly rectifying nature, the apical channels are largely involved in recycling K+ ions back to the SRS, a process that occurs because in RPE cells the resting membrane potential is more positive than the K equilibrium potential (EKeq), resulting in outward K+ currents. While Ba2+ blocks these Kir channels with different sensitivities, it is also a non-specific blocker of voltage-gated and Ca2+-activated K+ channels.
Thus, in the first experiment we tested this idea by investigating the interaction of the non-selective potassium channel blocker Ba2+ with the induction of refractive error by lens defocus.
Loop diuretics, by comparison, act to inhibit the cation-chloride NKCC cotransporter, thus inhibiting the coordinated trans-membrane movement of Na, K and Cl ions. Thus, in a second experiment, we tested the idea that if negative lens defocus results in myopia and ocular elongation through a reduction in the outflow of fluid across the RPE, then intravitreal injection of the diuretic bumetanide would restore RPE fluid flow as evidenced by relative suppression of abnormal vitreal chamber growth and inhibition of myopia.
Experiment 1: Potassium channels and refractive compensation
Potassium channels were blocked via intravitreal injection of barium chloride to a vitreal concentration of approximately 5mM. Retinoscopy and ultrasonography demonstrated that while saline injected eyes showed about 85% refractive compensation to the applied defocus over the four days of rearing, barium suppressed refractive compensation to both positive and negative lenses, but did not significantly affect refractive state for the chicks reared with focused vision (0D group).

The effect on refractive and growth compensation of blocking retinal and RPE potassium channels with intravitreal Ba2+ ion at an effective concentration of 5mM.
A. Scatter plot of Refraction in dioptres versus Vitreous Chamber depth (mm). across all eyes measured. The applied defocus is indicated by the triangles or circle symbols, with green symbols indicating Ba2+ and blue symbols indicating saline injected eyes. B. Refractions of eyes injected with Ba2+ compared with those injected with similar volume of Saline. Compensation in Ba2+ eyes is suppressed for both positive and negative lens defocus. The same colour code applies as for A. C. Vitreous chamber depths of Ba2+ and SAL eyes. An inverse relationship between vitreous chamber depth and refraction is evident. The same colour code applies as for A. The effect of defocus on VC depth is much less for Ba2+ than for SAL eyes, however the mean VC depth for Ba2+ eyes averaged over all lens groups is very similar to that for SAL eyes. This indicates that Ba2+ does not inhibit eye growth per se, but suppresses compensation to defocus-related eye growth.
Experiment 2: Effect of the loop diuretic bumetanide
In hatchling chicks, bumetanide suppressed refractive compensation to negative lenses in a dose specific fashion, but did not significantly affect refractive compensation to optical defocus of +10D. A scatter plot demonstrates the general negative linear relationship between refraction and vitreous chamber length.
At the higher concentration of bumetanide (Bum1: 10−5M), there was a significant difference in refractive compensation to negative lens defocus between the bumetanide and control Saline injected eyes (Bum1: −2.42±1.17 D, SAL: −8.59±0.93 D). Rearing with −10D lenses, the higher dose of bumetanide resulted in a sparing of over 6 D of refractive compensation compared with the Saline group, while the lower dose was markedly less effective (−5.3±0.74 D).
Indeed, further post-hoc testing within the separate lens rearing groups (see Table 2) indicates that bumetanide only significantly affects refractive state in the negative lens reared chickens.
Axial dimension of the vitreous chamber of the three negative lens wearing groups also show significant differences. Thus, eyes injected intravitreally with bumetanide, showed a slightly greater vitreal chamber depth after 4 days rearing than did eyes injected with the same volume of saline, measured over all Lens groups.
The general VC elongation seen with bumetanide (except for the higher dose with −10D lens rearing) was slightly unexpected. It appears that a model to explain the data should include a defocus independent elongation effect as well as a defocus dependent effect on refractive compensation and vitreous chamber depth that applies under conditions of negative lens defocus.

The effect on refractive compensation of the loop diuretic bumetanide at two concentrations in the vitreous chamber compared with control saline injections.
Bum1 refers to an effective vitreal concentration of 10−5 M, Bum2 refers to an effective concentration of 5×10−6 M. A. Scatter plot of Refraction in dioptres versus Vitreous Chamber depth (mm) across all eyes measured. The applied defocus is indicated by the triangles or circle symbols, with red filled symbols indicating Bum1, red open symbols indicating Bum2 and blue symbols indicating saline injected eyes. B. Compensation to negative lens rearing markedly diminished for both bumetanide doses with the higher dose (Bum1) resulting in approximately 6D less refractive compensation than for SAL. The same colour code applies as for A. The refractive effect is specific to the induction of myopia, with 0D and +10D groups showing no significant effect of the intravitreal diuretic. C. Vitreous chamber depths of bumetanide and saline injected eyes. The same colour code applies as for A. While the same trend as in A is evident with a dose dependency with negative lens defocus, it can also be seen that mean VC depth is slightly larger for Bumetanide cf SAL eyes, especially for 0D and +10D groups. Data presented as means±SE.
The effect of the trace element zinc on the change in the axial length and diopters and the variations of activities of superoxide dismutase (SOD) and nitric oxide synthase (NOS), and the content of NO in the retino pigmental epithelium choroid homogenate of the myopic eyes in form-sense-deprived chicks were studied. The results show that zinc can inhibit the elongation of axis oculi and increase the diopters in myopia. Meanwhile, the activities of SOD and NOS and the content of NO are significantly increased compared with the model group, indicating that zinc can be used to prevent and treat myopia to a certain extent.
Dopamine
In an attempt to understand the biological implications of our observations, we administered either apomorphine or haloperidol, a dopamine agonist and antagonist, respectively.
Although both are considered relatively nonselective, each shows somewhat greater affinity for the D2 compared with the D1 dopamine receptor subtype.
Apomorphine lessened the expected axial elongation of the lid-sutured eye in a dose-dependent fashion. At the highest dose (250 ng), apomorphine blocked lid-suture-induced axial elongation completely. Moreover, its effect was nullified by coadministration of the dopamine receptor antagonist, haloperidol, suggesting the involvement of dopamine receptors.
Haloperidol alone produced a partial decrease in axial elongation, statistically significant when the treatment groups were combined; however, its effect was neither dose dependent nor significant (P > 0.05) at any of the individual doses tested. We did not specifically evaluate whether these drugs influenced solely the axial dimension of the vitreous chamber or whether they also affected the much smaller anterior chamber. Most remarkably, all of the pharmacological treatments were selective; none influenced the exaggerated equatorial growth that takes place behind a lid suture.
Thus, deprivation of form vision in the newborn chick simultaneously perturbs ocular growth and retinal dopamine metabolism. Reduced retinal dopamine in deprived eyes is observable only during light adaptation and is associated with a decrease in tyrosine hydroxylation. Administration of pomorphine or haloperidol to an eye can reduce and sometimes even rectify the exaggerated axial growth that accompanies visual deprivation by lid suture.
