DEFINITION
Elastin is a structural protein that provides elasticity in connective tissues. Elasticity is especially important for blood vessels and lung tissues, which have an expectedly high elastin content.
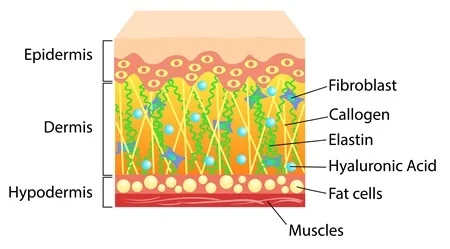

Molecular Mechanisms of Stress-Responsive Changes in Collagen and Elastin Networks in Skin , 2016
THE GENE
CHEMICAL STRUCTURE AND IMAGES
When relevant for the function
- Primary structure
- Secondary structure
- Tertiary structure
- Quaternary structure
Protein Aminoacids Percentage (Width 700 px)



SYNTHESIS AND TURNOVER
Downregulation
Ascorbate
Ascorbate Differentially Regulates Elastin and Collagen Biosynthesis in Vascular Smooth Muscle Cells and Skin Fibroblasts by Pretranslational Mechanisms 1997(19)78600-1/fulltext
- Ascorbate contributes to several metabolic processes including efficient hydroxylation of hydroxyproline in elastin, collagen, and proteins with collagenous domains, yet hydroxyproline in elastin has no known function. Prolyl hydroxylation is essential for efficient collagen production; in contrast, ascorbate has been shown to decrease elastin accumulation in vitro and to alter morphology of elastic tissues in vivo. Ascorbate doses that maximally stimulated collagen production (10-200 μM) antagonized elastin biosynthesis in vascular smooth muscle cells and skin fibroblasts, depending on a combination of dose and exposure time. Diminished elastin production paralleled reduced elastin mRNA levels, while collagen I and III mRNAs levels increased. We compared the stability of mRNAs for elastin and collagen I with a constitutive gene after ascorbate supplementation or withdrawal. Ascorbate decreased elastin mRNA stability, while collagen I mRNA was stabilized to a much greater extent. Ascorbate withdrawal decreased collagen I mRNA stability markedly (4.9-fold), while elastin mRNA became more stable. Transcription of elastin was reduced 72% by ascorbate exposure. Differential effects of ascorbic acid on collagen I and elastin mRNA abundance result from the combined, marked stabilization of collagen mRNA, the lesser stability of elastin mRNA, and the significant repression of elastin gene transcription.
Smoke
smoke+and+elastin
UV
uv+and+skin+elastin
mRNA synthesis
protein synthesis
post-translational modifications
degradation
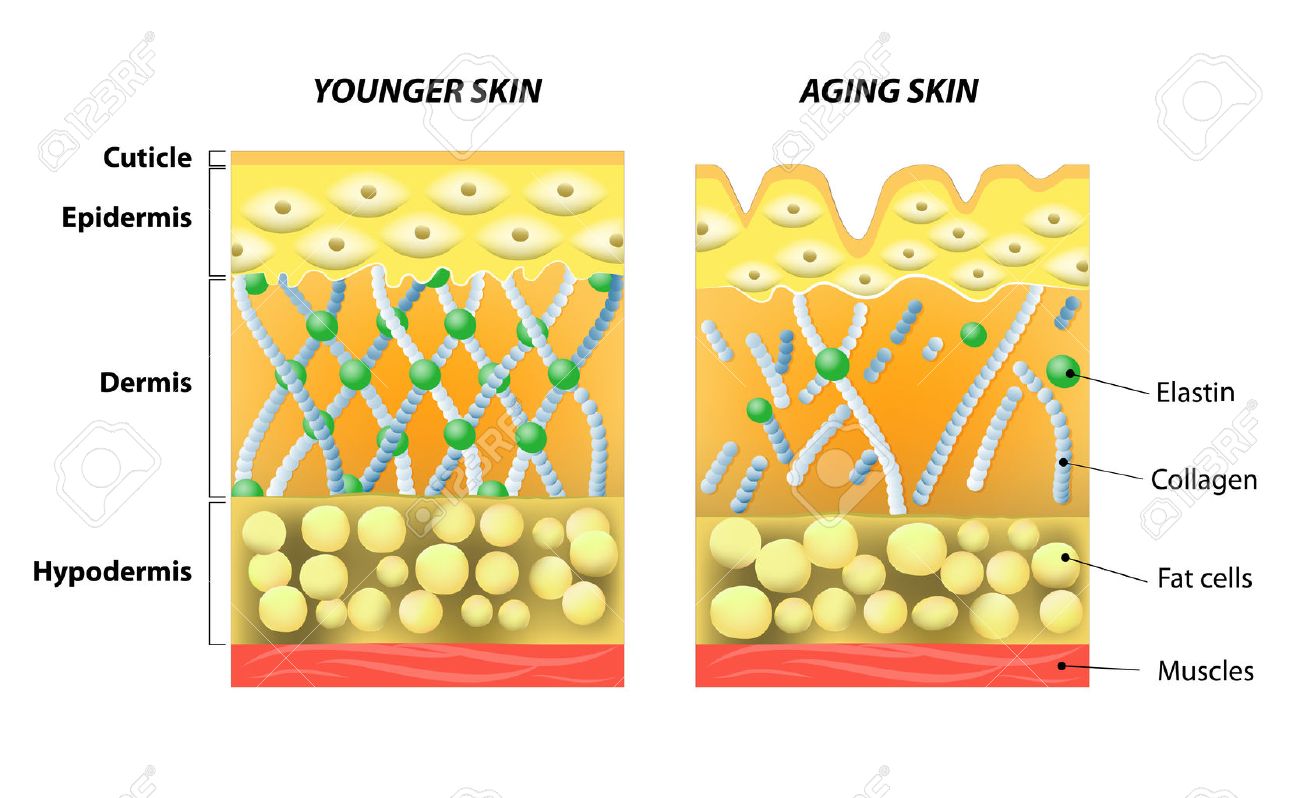
CELLULAR FUNCTIONS
cellular localization,
It is classed as a fibrous protein because of its structural function and relative insolubility in water.
Elastin lacks a regular secondary structure. As in collagen, allysines form cross-links in elastin. An extracellular lysine amino oxidase converts lysine side chains in the sequence –Lys-Ala-Ala-Lys and –Lys-Ala-Ala-Ala-Lys to allysines. Three allysines and an unmodified lysine from different regions in the polypeptide chains react to form the heterocyclic structure of desmosine or isodesmosine, which cross-links the polypeptide chains in elastin networks.
The amino-acid composition of elastin is rich in proline and glycine, like that of collagen, but elastin does not have glycine as every third residue, nor does it have a triple-helical structure.
Instead, elastin is rich in alanine and valine.
A prototypical elastin sequence is Val–Pro–Gly–Val–Gly, and peptides composed of (Val–Pro–Gly–Val–Gly)n repeats are substrates for P4H.
The creation of Hyp in elastin is catalyzed by the collagen P4H, but there is less Hyp in elastin than in collagen and Hyp is not required for elastin biosynthesis or secretion
|  |
Proline in elastin-like polymers is efficiently hydroxylated under conditions where these polymers exhibit preferred secondary and tertiary structures.
biological function
h4 Elastin and Emilin 1, 2, 3



The EMILIN/multimerin family, 2011
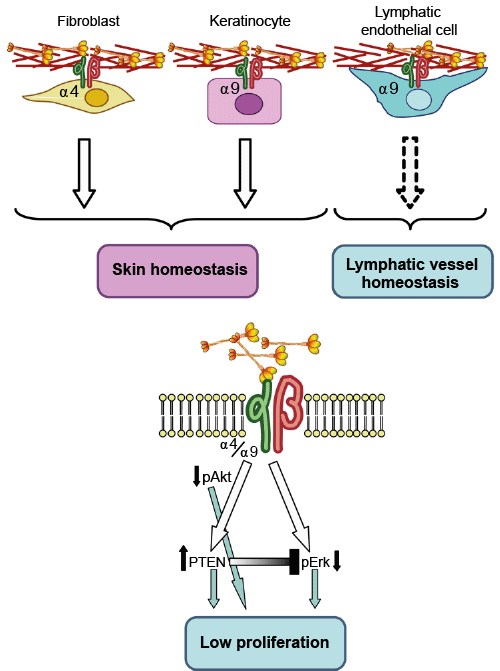
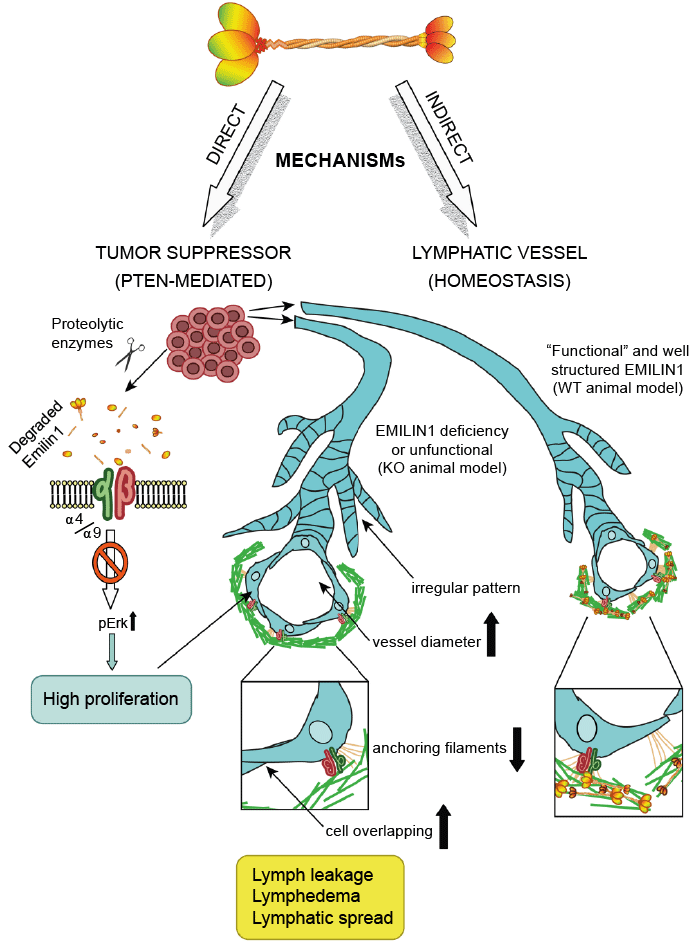


Elastin related enzymes AA
- Cell signaling and Ligand transport
- Structural proteins
REGULATION
DIAGNOSTIC AND THERAPEUTIC USES
Clinical Relevance of Elastin in the Structure and Function of Skin, 2021
- Abstract
Elastin is the main component of elastic fibers, which provide stretch, recoil, and elasticity to the skin. Normal levels of elastic fiber production, organization, and integration with other cutaneous extracellular matrix proteins, proteoglycans, and glycosaminoglycans are integral to maintaining healthy skin structure, function, and youthful appearance. Although elastin has very low turnover, its production decreases after individuals reach maturity and it is susceptible to damage from many factors. With advancing age and exposure to environmental insults, elastic fibers degrade. This degradation contributes to the loss of the skin’s structural integrity; combined with subcutaneous fat loss, this results in looser, sagging skin, causing undesirable changes in appearance. The most dramatic changes occur in chronically sun-exposed skin, which displays sharply altered amounts and arrangements of cutaneous elastic fibers, decreased fine elastic fibers in the superficial dermis connecting to the epidermis, and replacement of the normal collagen-rich superficial dermis with abnormal clumps of solar elastosis material. Disruption of elastic fiber networks also leads to undesirable characteristics in wound healing, and the worsening structure and appearance of scars and stretch marks. Identifying ways to replenish elastin and elastic fibers should improve the skin’s appearance, texture, resiliency, and wound-healing capabilities. However, few therapies are capable of repairing elastic fibers or substantially reorganizing the elastin/microfibril network. This review describes the clinical relevance of elastin in the context of the structure and function of healthy and aging skin, wound healing, and scars and introduces new approaches being developed to target elastin production and elastic fiber formation.
elastin covid-19