IS ALCOHOL TO CAUSE PSORIASIS OR PSORIASIS CAUSES ALCOHOLISM?
Psoriasis and alcohol: is cutaneous ethanol one of the missing links?, 2010
Alcohol intake and risk of incident psoriasis in US women: A prospective study, 2010
Alcohol intake: a risk factor for psoriasis in young and middle aged men?, 1990
Alcohol as a risk factor for plaque-type psoriasis, 2005
Alcohol consumption and psoriatic risk: a meta-analysis of case-control studies, 2012
What is psoriasis and how could it be connected to alcoholism?
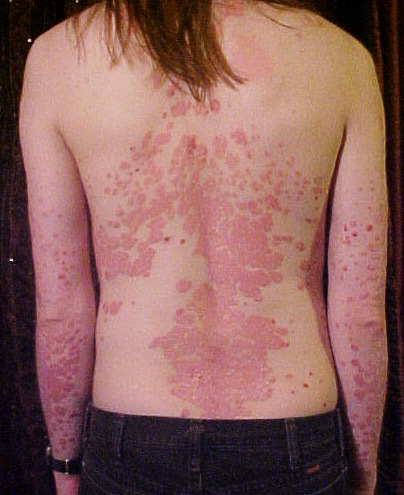
Psoriasis is a chronic inflammatory skin disorder mediated by lymphocytes T. Genetic factors are essential in the development of the disease but can be involved certain environmental factors. Environmental triggers that can trigger or exacerbate psoriasis include endocrine factors, infections, trauma, psychological stress, obesity, smoking and alcohol abuse.
Environmental factors and psoriasis, 2007
Alcohol consumption is significantly higher in patients with psoriasis than in healthy controls, but there often arises the question: "is the alcohol to cause psoriasis or psoriasis causes alcoholism?". A recent study showed a small but significant association between alcohol consumption and anxiety and depression of psoriatic patients, so it is possible that these individuals are using alcohol to combat these disorders.
Alcohol consumption and psychological distress in patients with psoriasis, 2008
On the other hand, there is an evidence that ethanol could directly worsen or get precipitate psoriasis through various mechanisms. Drinking is not associated with therapeutic compliance and the abuse of alcohol may reduce the effectiveness and increase the toxicity of systemic treatments as psoralens, retinoids, methotrexate and cyclosporine. In the presence of ethanol the possible esterification of retinoid acitretin in etretinate has been described and in patients with psoriasis a trend that relates a higher consumption of alcohol with an increased risk of synthesis of etretinate and with a higher level of this molecule has been observed. Conversion of acitretin to etretinate in psoriatic patients is influenced by ethanol, 1993
As etretinate is eliminated slowly and accumulates in adipose tissue, it is possible that alcohol abuse increases the potential teratogenic and the possible side effects of oral acitretin.
Alcohol changes the immune system
Ethanol can influence the immune system in various ways concerning specific functions, cells and molecules, both innate immunity and adaptive. In general the alcohol inhibits the inflammatory and immune responses;however, the consumption of alcohol acute and chronic has opposite effects on inflammatory cell activation. It seems that acute alcohol exposure has an inhibiting effect, while chronic causes an increase in inflammatory cell responses.
Clinical and experimental data that may explain how ethanol could contribute to the development of psoriasis were collected. It is known that alcoholics are more prone to infections.
Because of streptococcal infections are factors that trigger psoriasis, this increased susceptibility may be involved in the onset and progression of disease.
Alcoholism and psoriasis-an immunological relationship, 1991
Recent developments in alcoholism:immunological aspects, 1993
Chronic consumption of alcohol increases the expression of co-stimulating molecules (CD80 and CD86.) on splenocytes activated by toll-like receptor 9 that leads to a systemic immune disruption including activation of lymphocytes T. Chronic ethanol ingestion by mice increases expression of CD80 and CD86 by activated macrophages, 2004
In this connection, monocytes and macrophages in peripheral blood of patients with alcoholic liver disease show an increased production of tumor necrosis factor (TNF-α) with activation of the inflammatory cascade pathway.
The TNF-α-mediated flogosis plays an important role in psoriasis. The TNFα is initially produced as a transmembrane protein. From this integrated membrane form, the soluble cytokine is released via proteolytic cleavage by the converting enzyme of TNFα (TACE). In psoriatic lesions and in peripheral blood mononuclear cells of patients with psoriasis triggers, there is an overexpression of TACE and in vivo was demonstrated that excessive consumption of ethyl alcohol contributes to the up-regulation of the expression of TACE in peripheral blood mononuclear cells. Overexpression of tumour necrosis factor-alpha-converting enzyme in psoriasis, 2005
This causes an increase in blood-1 receptor of the transforming growth factor soluble, TGFα, another marker of active psoriasis.
Tumour necrosis factor (TNF-alpha) alpha converting enzyme and soluble TNF-alpha receptor type 1 in psoriasis patients in relation to the chronic alcohol consumption, 2008
It is well known that T-cells activated by an overgrowth of keratinocytes are key features of psoriasis. For this, a model of co-culture with keratinocytes psoriasis and a line of T lymphoma cells (HUT-78) has been created. In this co-culture system, adding ethanol (0.05%) the level of Interleukin-6, TGFalfa and IFNgamma interferon increased, which was of significant increased response type 1 helper T (Th1).
Another study has shown that ethanol stimulates a marked lymphocytic proliferation induced by mitogens in patients with psoriasis, which may also occur in vivo.
Ethanol enhances the IFN-gamma, TGF-alpha and IL-6 secretion in psoriatic co-cultures, 1996
Ethanol enhances the mitogen-driven lymphocyte proliferation in patients with psoriasis, 1996
Ethanol and acetone stimulate the proliferation of keratinocytes
Acetone is derived from the decarboxylation of acetoacetic acid, a precursor which is itself derived from Acetyl Coenzyme A. An increased synthesis of the reduced form of nicotinamide adenine dinucleotide, directly connected to the ethanol metabolism, slows the degradation of Acetyl Coenzyme A and increases levels of acetone in blood; as a result, heavy drinkers show elevated plasma concentrations of acetone. The effects of ethanol and acetone have been studied on proliferation of keratinocytes line called HaCaT. The HaCaT are cultivated and incubated with different concentrations of ethanol and acetone and it has been observed that both increase vitality and proliferation of keratinocytes. The greatest increase in the number of viable cells and proliferative response occurres at doses of 0.0025% ethanol and acetone 0.0079%. It has been also shown that ethanol and acetone up-regulate genes mRNA levels typical of keratinocytic proliferation and the receptor for the keratinocyte growth factor. For this reason, it was described that the stimulatory effect of acetone and ethanol on human keratinocytes can be one of the reasons that psoriasis can be triggered by alcohol abuse.
Ethanol and acetone stimulate the proliferation of HaCaT keratinocytes: the possible role of alcohol in exacerbating psoriasis, 2003
Vitamin D and psoriasis
Photochemical synthesis of vitamin D3 (cholecalciferol, D3) occurs cutaneously where pro-vitamin D3 (7-dehydrocholesterol) is converted to pre-vitamin D3 (pre-D3) in response to ultraviolet B (sunlight) exposure.
DHCR7 encodes the enzyme 7-dehydrocholesterol (7-DHC) reductase, which converts 7-DHC to cholesterol, thereby removing the substrate from the synthetic pathway of vitamin D3, a precursor of 25-hydroxyvitamin D3.
Vitamin D3, obtained from the isomerization of pre-vitamin D3 in the epidermal basal layers or intestinal absorption of natural and fortified foods and supplements, binds to vitamin D-binding protein (DBP) in the bloodstream, and is transported to the liver.
D3 is hydroxylated by liver 25-hydroxylases (25-OHase).
The resultant 25-hydroxycholecalciferol (25(OH)D3) is 1-hydroxylated in the kidney by 25-hydroxyvitamin D3-1 -hydroxylase (1-OHase).
This yields the active secosteroid 1,25(OH)2D3 (calcitriol), which has different effects on various target tissues.
Vitamin D activity is mediated through binding of 1,25(OH)2D3 to the vitamin D receptor (VDR), which can regulate transcription of genes involved in cell regulation, growth, and immunity.
What's the relationship between the liver and Vitamine D? The liver is one the tissues involved in Vitamine D synthesis. Alcohol abuse normally provokes the alcoholic liver disease, a hepatic manifestations of alcohol overconsumption, including fatty liver, alcoholic hepatitis, and chronic hepatitis with hepatic fibrosis or cirrhosis. The consequence is the loss of hepatocyte functions, including the ability to convert D3 in 25OHD3.
What's the relationship between Vitamine D and psoriasis? It has been studied that Vitamine D is involved in Cathelicidins regulation, an important Antimicrobial peptide (AMP) in the skin. Human cathelicidin is often referred to by one of its peptide forms (LL-37).
The vitamin D pathway: a new target for control of the skin’s immune response?
Cathelicidins, like many AMPs, are produced in keratinocytes, neutrophils, and many other cell types.
In initial observations cathelicidin expression in skin followed a pattern that was expected for a molecule involved in defense function. Cathelicidin expression is high in bacterial skin infection and induced by cutaneous barrier disruption such as in invasive bacterial infection or physical injury of the skin; moreover it has been shown an abnormal AMPs activity associated with psoriasis.
Cathelicidin is increased in lesional skin in psoriasis. Endogenous antimicrobial peptides and skin infections in atopic dermatitis, 2002
Recent studies showed that LL-37 isolated from lesional skin was shown to form complexes with human self-DNA to activate plasmocytoid dendritic cells (pDCs). pDCs do not normally respond to self-DNA but binding to LL-37 converted DNA in a potent stimulus for pDC activation. LL-37/self-DNA complexes signalled through TLR9 and elicited IFN-α release from pDCs. IFN-α subsequently activated a T-cell response that can lead to cutaneous inflammation.
Antimicrobial peptides and the skin immune defense system, 2008
Vitamin D is one of the most important response element in the cathelicidin promoter; Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression, 2004 ; several research groups also confirmed that cathelicidin is a direct target of vitamin D3 in keratinocytes.
Additional elements of the vitamin D3 signalling cascade have been identified that lead to increased cathelicidin such as recruitment of coactivators or epigenetic changes.
Still, it was unclear how cathelicidin is induced in bacterial infections, in wounds or in chronic inflammatory skin disorder, situations where a sudden change in 1,25-dihydroxy vitamin D3 levels seemed unlikely. The solution to this dilemma came with recognition that 1α-hydroxylase (CYP27B1) executes a hydroxylation step in the skin that generates the biologically active form of vitamin D3 (1,25D3).
This activation step for vitamin D3 occurs in monocytes and keratinocytes by CYP27B1 and is under the control of inflammatory stimuli combined with TLR2. Upon skin injury or bacterial infection there is a local increase in expression of CYP27B1 and as a direct consequence more vitamin D3 is activated to induce cathelicidin expression and function.
Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D–dependent mechanism, 2007
Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, 2006
Finally, in psoriasis, peptide cathelicidin block could break the vicious cycle of increased expression of LL-37, pDC activation and skin inflammations. Strategies to decrease cathelicidin in keratinocytes may purpose signalling of vitamin D3. Paradoxically, for a long time, vitamin D3 analogues have been used in the treatment of psoriasis. Vitamin D3 analogues bind and activate vitamin D receptor and therefore should increase cathelicidin in keratinocytes likely worsening inflammation in psoriasis. However, the opposite is true: vitamin D analogues resemble one of the pillars of topical psoriasis treatment. They improve the cutaneous inflammation and reverse the morphological changes within the skin lesion.
Combination therapy to treat moderate to severe psoriasis, 2004
Calcipotriol and psoriasis topic treatment with Vitamin D
The vitamin, directly or indirectly, controls more than 200 genes, including those responsible for the regulation of cell proliferation, differentiation, apoptosis and angiogenesis. It therefore reduces the proliferation of normal cells, but also of cancerous ones and induces their terminal differentiation. To this effect it is also practically used in the treatment of psoriasis. In addition, as the 1.25(OH)vitD3 can induce differentiation and inhibit the proliferation of normal and malignant cells, its lack (as well as alcoholic liver disease) should be associated with an increased risk for almost all major human diseases such as cancer, autoimmune diseases, cardiovascular and metabolic ones.
Treatment of Psoriasis
Psoriasis continues to be one of the more difficult skin conditions to treat. The wide range of treatments available for psoriasis illustrates this; no one treatment will work for everyone. There is no cure for psoriasis but several new medications have recently been introduced and ongoing research looks promising. In general the treatment is chosen on the basis of the pattern of psoriasis and its severity. Sometimes several treatments may need to be tried before the most suitable regime is established. Different medications may need to be used together or in rotation for best effect or to minimize side effects. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet®/Daivobet®/Taclonex®) in the treatment of psoriasis vulgaris, 2006
Calcipotriol is belongs to the group of medicines known as vitamin D analogues used to treat plaque psoriasis. Plaque psoriasis is a skin disorder caused by cells in the outer layer of the skin multiplying too quickly. As new skin cells are produced, old ones are shed. If this process is taking place too quickly, old skin cells build up on the skin surface causing red, scaly patches. Calcipotriol helps to control psoriasis by slowing down the production of new skin cells. Currently, calcipotriol (a synthetic vitamin D3 analog) is one of the most popular topical treatments. Transcriptional profiling of keratinocytes reveals a vitamin D-regulated epidermal differentiation network, 2005
Mode of action of Calcipotriol
Calcipotriol binds to be specific intracellular receptors present in the keratinocytes.The receptor- vitamin- D complex binds to a specific gene the DNA that modulates and controls transcription. The receptor is present in human epidermal keratinocytes, dermal fibrinoblasts and lymphocytes. At appropriate concentration, calcipotriol causes a decrease in the proliferation and an increase in the morphologic and biochemical differentiation of keratinocytes, hence regulate their proliferation and differentiation. Calcipotriol competes with calcitriol in binding with these receptors when applied topically over a psoriatic skin.
Cutaneous ethanol is one of the missing links?
It is well known that the amount of alcohol ingested are secreted through the skin.
Transdermal ethanol derives from two processes: the active secretion by eccrine glands, sweat glands, especially and passive diffusion through the epidermal lipid leaflets.
Ethanol increases skin permeability to many chemicals agents, damaging the epidermal barrier, and increases the solubility of penetration of chemical compounds; for this reason, in rats was examined transdermal penetration of different chemicals after acute ethanol consumption. in these experiments were calculated and skin blood concentrations of alcohol after a single intake. There is a dose-dependency between ethanol administered orally and plasma concentrations (after 2 hours the levels of ethanol in the skin were equal to 12-18% of those in blood) so these ethanol concentration ranges measured in rats after 2 hours from alcohol intake can be overlapped to the values that in vitro cause the proliferation of keratinocytes and those that increase lymphocyte proliferation in patients with psoriasis.
The correlation in humans between ethanol concentrations in plasma and sweat has been examined in a study. This correlation was very strong (r = 0.97) and the slope of the relationship was 1.01. This result indicates that ethanol is balances quickly between the sweat and the blood and these concentrations were proportional to each other.
Sweat ethanol concentrations are highly correlated with co-existing blood values in humans, 1999
The first commercial product which makes use of transdermal alcohol assessment was a sweat-patch. Numerous studies have been performed with this device and it has been established that there is a statistically significant linear association between the concentration of ethanol in the sweat and blood that mean.
Subjective responses to the sweat-patch test for alcohol consumption, 1984
While studies on sweat-patch focused on ethanol concentrations in sweat, other, conducted in the late ' 80, have assessed the concentrations of ethanol in the steam that forms on the skin. Although the total amount of ethanol deleted through perspiration is about 1%, concurrent estimates of ethanol contained in perspiration showed concentrations roughly similar to those of the blood and other body fluids.
A method for determining the excretion of volatile substances through skin, 1985
Ethanol vapor above skin: determination by a gas sensor instrument and relationship with plasma concentration, 1987
In 1990 the wristbands Wrist Transdermal Alcohol Sensor were created to determine the transdermal alcohol concentration (TAC). Several measures showed that, after taking drinks with high alcohol content, transdermal alcohol peaks are found. These TAC values are in the range of concentration which increases the proliferation of keratinocytes in vitro, increases the synthesis of Th1 cytokines and induces lymphocyte proliferation in patients with psoriasis.
CONCLUSION
In conclusion, precise determination of alcohol consumption is important for application in legal, clinical and experimental field. It is known that ethanol ingested is excreted through the human skin and that its concentration in perspiration is almost similar to that in the blood and other body fluids. In animals and in humans, several methods were used to evaluate the TAC, as the determination in sweat, in epidermis and in the steam. The creation of alcohol monitoring devices in the form of bracelets that measure the concentration of ethanol in the steam produced on the skin makes possible the noninvasively determination of TAC. All these evaluations have shown that the levels of alcohol in the epidermis correspond to the ones in blood and breath and that TAC values are into the range of those which stimulate the keratinocytic proliferation, increase the synthesis of cytokines Th1 and lymphocyte proliferation.
Observation that alcoholics are more predisposed to various infections and that ethanol affects the epidermal barrier function may partly explain his role in the development of psoriasis. The ethanol-induced synthesis of Th1 cytokine and lymphocyte and keratinocytic proliferation in the epidermis can be additive factors in the pathogenesis of the disease.
Acute exposure to alcohol inhibits inflammatory responses, while chronic increases them.
Also the various parameters of an alcoholic may be important, such as body weight, metabolic rate (including the skin) and the amount of alcohol. There is also a time interval between the values of BAC (blood alcohol concentration) and those of TAC and it seems that ethanol can stay longer in the epidermis than that in the blood or breath. Different skin sites can exhibit various levels of ethanol depending on the thickness of the stratum corneum and density of hair follicles and sweat glands, thus leading to higher concentrations and lower, which may also have a clinical relevance.
Levels of acetone and other alcohol metabolites of the human epidermis are not known; it is therefore difficult to assess their clinical importance in the induction of psoriasis.
In summary, it is expected that the development of techniques for determining the TAC will provide additional tools to doctors for evaluating how alcohol affects the physiology of the skin and various skin conditions, including iperproliferative diseases such as psoriasis.