DEFINITION
The carbonic anhydrases (or carbonate dehydratases) form a family of enzymes that catalyze the rapid interconversion of carbon dioxide and water to bicarbonate and protons (or vice versa), a reversible reaction that occurs rather slowly in the absence of a catalyst.
Carbonic anhydrase II is a monomeric cytosolic isoenzyme of carbonic anhydrase with no posttranslational modification. Its molecular mass is 29 kDa. (Carbonic anhydrase II deficiency: single-base delection in exon 7 is the predominant mutation in Caribbean Hispanic Patients, 1994)
THE GENE
The gene for CAII was mapped to chromosome 8q22.
It's 20 kb long and contains 7 exons.
(The neurology of carbonic anhydrase type II deficiency syndrome, 2011)
The three cytoplasmic carbonic anhydrase isozymes CAI, CAII, and CAIII lie on the long arm of chromosome 8 (8q22) in humans. The genes lie in the order CA2, CA3, CA1. CA2 and CA3 are separated by 20 kb. CA1 is separated from CA3 by over 80 kb. (Physical mapping of the human carbonic anhydrase gene cluster on chromosome 8, 1991)

CHEMICAL STRUCTURE AND IMAGES
The CA II structure can be described as a single domain mixed α/β globular protein. The central structural motif is a twisted β-sheet of 8 strands, which is near seven α-helices. The catalytic active site is characterized by a conical cleft that is approximately 15 Å deep with the zinc residing in the interior. The zinc is tetrahedrally coordinated by three histdine ligands (His 94, His 96, and His 119) and a bound water/hydroxyl. The zinc-ligand distances are all ∼2.1 Å including the zinc-bound solvent molecule.
There is a hierarchy of zinc ligands in the active site: the first-shell, or direct zinc ligands, are the three histidine residues His 94, His 96, His 119 and a solvent molecule. The second-shell, or indirect ligands, stabilize the direct ligands and help position them for zinc ion coordination. Residue Gln 92 stabilizes His 94, Glu 117 stabilizes His 119, and the backbone carbonyl oxygen of Asp 244 stabilizes His 96, while residue Thr 199 hydrogen bonds with the zinc-bound solvent. Finally a third-shell of stabilization was proposed of a cluster of aromatic residues (Phe 93, Phe 95, and Trp 97) that anchor the β-strand βF that contains His 94 and His 96.
(Apo-human carbonic anhydrase 2 revisited: implications of the loss of a metal in protein structure, stability and solvent network, 2009)
 PDB
PDB
Protein Aminoacids Percentage

CELLULAR FUNCTIONS
There are at least five distinct CA families (α, β, γ, δ and ε). The α-CAs are found in humans.
CAII is one of the α-CA family's enzyme.
The CA enzymes found in mammals are divided into four broad subgroups, which, in turn consist of several isoforms:
* Cytosolic CAs (CA-I, CA-II, CA-III, CA-VII and CA XIII)
* Mitochondrial CAs (CA-VA and CA-VB)
* Secreted CAs (CA-VI)
* Membrane-associated CAs (CA-IV, CA-IX, CA-XII, CA-XIV and CA-XV)
There are three additional "acatalytic" CA isoforms (CA-VIII, CA-X, and CA-XI) whose functions remain unclear.
CA-I is localized in red blood cell and GI tract.
CA-II is the most ubiquitous and the most studied. Due to its localization it is involved in a lot of biological process; therefore CA-II deficiency is linked to a syndrome that involves more than one organ.
CA-III is strictly tissue-specific and present at high levels in skeletal muscle and much lower levels in cardiac and smooth muscle.
CA-IV is a glycosylphosphatidyl-inositol-anchored membrane isozyme expressed on the luminal surfaces of pulmonary (and certain other) capillaries and of proximal renal tubules. Its exact function is not know.
CA-VA is expressed in liver.
CA-VB has a wider tissue distribution than CA-VA.
CA-VI is found in salivary glands and saliva.
CA-VII is widely distributed.
CA-IX is a transmembrane protein and is a tumor-associated carbonic anhydrase isoenzyme. It is considered to be one of the best cellular biomarkers of hypoxia.
CA-XII is highly expressed in normal tissues, such as kidney, colon and pancreas, and has been found to be overexpressed in 10% of clear cell renal carcinomas.
CA-XIII is widely distributed.
CA-XIV is expressed in kidney, heart, skeletal muscle, brain.
Biological Function
Carbonic anhydrase type II has the highest catalytic activity for physiological functions including electrolyte and water balance; pH homeostasis; CO2 and HCO3 transport; and production of aqueous humor, cerebrospinal fluid, gastric acidity and pancreatic secretions. It also assists in metabolic pathways such as gluconeogenesis, lipogenesis, ureagenesis and bone resorption and calcification. (The neurology of carbonic anhydrase type II deficiency syndrome, 2011)

It has a role in the gastric protonic secretion, thanks to its interaction with the Anion Exchanger 1.
In the proximal tubule the CAII works for the bicarbonate's uptake.
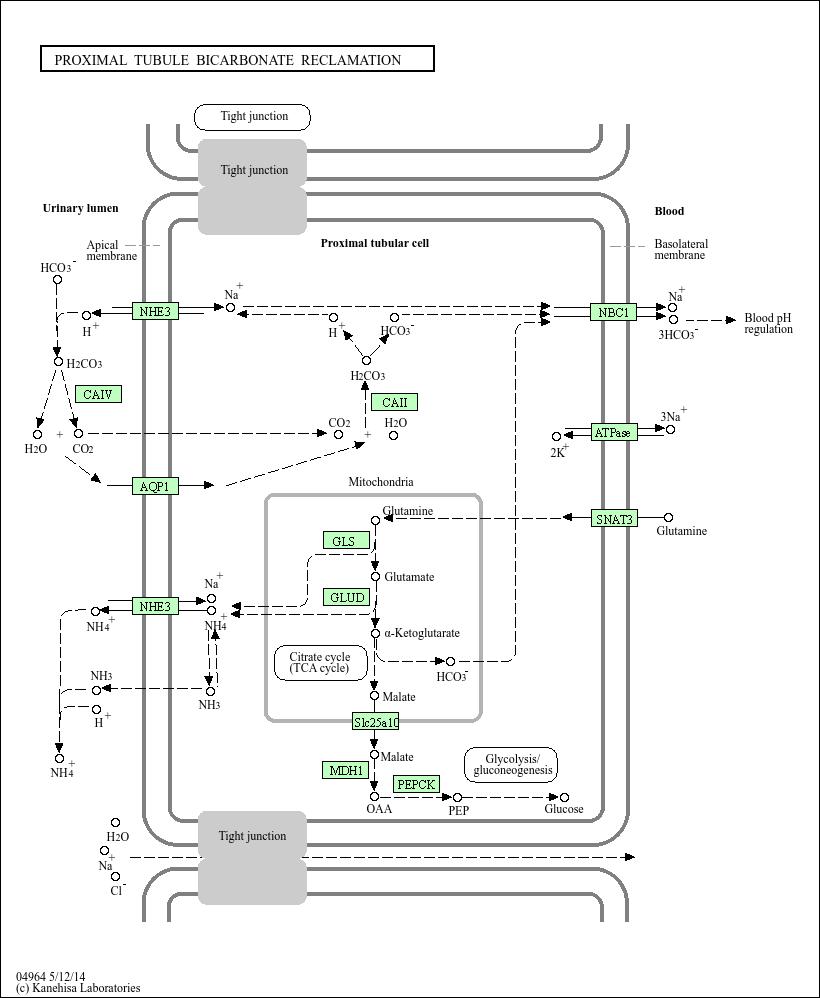
REGULATION
CAs are inhibited primarily by two main classes of compounds: the metal complexing inorganic anions ( cyanide, cyanate, thiocyanate, azide, hydrogensulphide etc.) and the sulphonamides. Inhibitors of both types directly bind to the metal ion within the enzyme active site. Sulphonamide CA inhibitors (CAIs) are useful as diuretics, or in the treatment and prevention of a variety of diseases such as glaucoma, epilepsy, congestive heart failure, mountain sickness, gastric and duodenal ulcers, neurological disorders and osteoporosis. (Carbonic anhydrase inhibitors and their therapeutic potential, 2000)
CAII DEFICIENCY SYNDROME
Carbonic anhydrase 2 deficiency syndrome (CADS) is an uncommon autosomal recessive disease with cardinal features including osteopetrosis, renal tubular acidosis and brain calcification. Other features of the disease include short stature, a large cranial vault, multiple skeletal fractures, developmental delay and cognitive defects varying from mild learning disabilities to severe mental retardation, anaemia, splenomegaly and secondary erythropoiesis.
Mutation
Three different structural gene mutations have been identified in patients with CADS. These include a missense mutation (H107Y), a splice Junction mutation in intron 5 (G-to-C) and a splice Junction mutation in intron 2(Arabic Mutation).
The Arabic Mutation is a splice site mutation from G to A in intron 2 of CA2 resulting in a crypting codon sequence followed by a premature stop codon that truncates the resultant complementary DNA.
Mental retardation is reported to be almost universal with this mutation.

In the H107Y mutation the C to T transition causes the histidine at position 107 to be replaced by a tyrosine.
This mutation doesn't compromize totally the activity of CAII. The residual activity may explain the absence of mental retardation.
(The neurology of carbonic anhydrase type II deficiency syndrome, 2011)
(Molecular bases of Human CAII deficiency, 1992)
Osteopetrosis
Osteopetrosis is a rare skeletal condition characterized by skeletal sclerosis caused by aberrant osteoclast-mediated bone resorption. Complications of osteopetrosis include increased susceptibility to fractures and cranial nerve compression symptoms. Anemia and other hematologic manifestations of osteopetrosis are absent.
(Osteopetrosis, 2003)

Renal tubular acidosis
Renal tubular acidosis is a medical condition that involves an accumulation of acid in the body due to a failure of the kidneys to appropriately acidify the urine.
Brain calcification
Brain calcification are generally progressive and follow a distinct distribution, involving predominantly basal ganglia and thalami and grey–white matter junction in frontal regions more than posterior regions.
At the grey–white matter junction, frontal lobes are involved more often than parietal, which in turn are involved more often than temporal lobes.
(The neurology of carbonic anhydrase type II deficiency syndrome, 2011)
AUTHORS
Irene Politi
Federica Valentino