Abdrirashid Mohamed
Enrico Ruffino

DESCRIPTION
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is an active component of chili peppers, which are plants belonging to the genus Capsicum. It is an irritant for mammals, including humans, and produces a sensation of burning in any tissue with which it comes into contact. Capsaicin and several related compounds are called capsaicinoids and are produced as secondary metabolites by chili peppers, probably as deterrents against mammals and fungi. Pure capsaicin is a volatile, hydrophobic, colorless, odorless, crystalline to waxy compound.
Capsaicin is present in large quantities in the placental tissue (which holds the seeds), the internal membranes and, to a lesser extent, the other fleshy parts of the fruits of plants in the genus Capsicum. The seeds themselves do not produce any capsaicin, although the highest concentration of capsaicin can be found in the white pith of the inner wall, where the seeds are attached.
NATURAL FUNCTION
The seeds of Capsicum plants are dispersed predominantly by birds: in birds, the TRPV1 channel does not respond to capsaicin or related chemicals (avian vs mammalian TRPV1 show functional diversity and selective sensitivity). This is advantageous to the plant, as chili pepper seeds consumed by birds pass through the digestive tract and can germinate later, whereas mammals have molar teeth which destroy such seeds and prevent them from germinating. Thus, natural selection may have led to increasing capsaicin production because it makes the plant less likely to be eaten by animals that do not help it reproduce.
There is also evidence that capsaicin may have evolved as an anti-fungal agent: the fungal pathogen Fusarium, which is known to infect wild chilies and thereby reduce seed viability, is deterred by capsaicin, which thus limits this form of predispersal seed mortality.
Wikipedia, Capsaicin Natural Function
CAPSAICIN PHARMACOLOGY
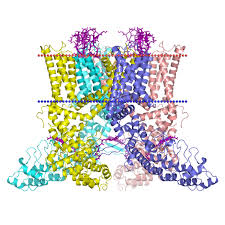
Capsaicin is a highly selective and potent (low nanomolar affinity) exogenous agonist for the TRPV1 receptor, a trans-membrane receptor-ion channel complex which provides integrated responses to temperature, pH, and endogenous lipids. Temperatures of 43°C or higher or acidity of pH of <6.0 can directly activate the channel, but combinations of these two stimuli can activate the channel at substantially lower temperatures or pH values.
Numerous putative endogenous agonists for TRPV1 have been identified; these include anandamide, N-acyldopamines, other long-chain unsaturated fatty acids, and lipoxygenase compounds such as leukotriene B4 and 12-(S) and 15-(S)-hydroperoxyeicosatetraenoic acid. Recently, oxidized metabolites of linoleic acid have been added to the list of potential endogenous agonists. Responsiveness of TRPV1 receptors to these activators is also highly regulated by the phosphorylation state of the channel complex, the presence of ancillary proteins, and an ever-growing number of allosteric modifiers.
When activated by a combination of heat, acidosis, or endogenous/exogenous agonists, TRPV1 may open transiently and initiate depolarization mediated by the influx of sodium and calcium ions. In the nociceptive sensory nerves which selectively express TRPV1 (mostly C- and some Aδ-fibres), depolarization results in action potentials, which propagate into the spinal cord and brain, and may be experienced as warming, burning, stinging, or itching sensations.
TRP channels and pain, 2009
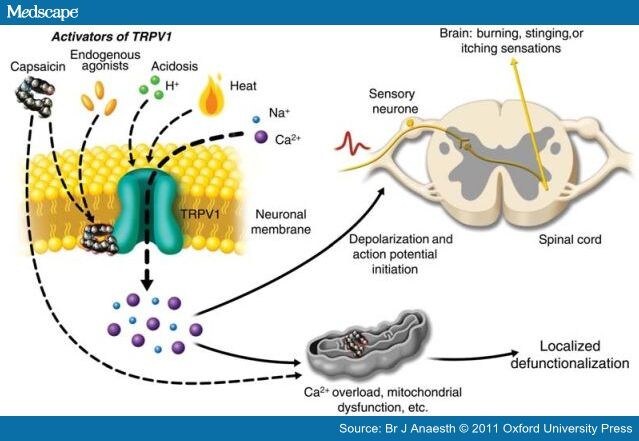
In contrast to transient activation which follows normal environmental stimuli or inflammatory responses to tissue injury, activation of TRPV1-expressing nerve fibres by exposure to a chemically stable exogenous agonist, such as capsaicin, can generate a biochemical signal with a persistent effect. The TRPV1 channel is highly calcium permeable (with a calcium:sodium permeability ratio that starts at about 8:1 and increases to about 25:1 during prolonged capsaicin exposures), which allows significant amounts of calcium to flow down its steep electrochemical gradient into nerve fibres.
TRPV1 shows dynamic ionic selectivity during agonist stimulation,2008
Furthermore, as TRPV1 is also expressed on intracellular organelles, external capsaicin application can cause release of calcium from the endoplasmic reticulum and induce additional intracellular calcium release from internal stores via calcium-dependent calcium release
Consequently, sustained high levels of intracellular calcium can activate calcium-dependent enzymes such as proteases, and can induce the depolymerization of cytoskeletal components such as microtubules.
An additional effect of high concentrations of capsaicin, which does not involve TRPV1, is a direct inhibition of mitochondrial respiration.
TRPV1-expressing sensory nerve fibres are exposed to high concentrations of capsaicin or to lower concentrations in a continuous fashion, high levels of intracellular calcium and the associated enzymatic, cytoskeletal, and osmotic changes, and the disruption of mitochondrial respiration lead to impaired local nociceptor function for extended periods.
Transient receptor potential vanilloid subtype 1 channel mediated neuropeptide secretion and depressor effects: role of endoplasmic reticulum associated Ca2+ release receptors in rat dorsal root ganglion neurons,2008
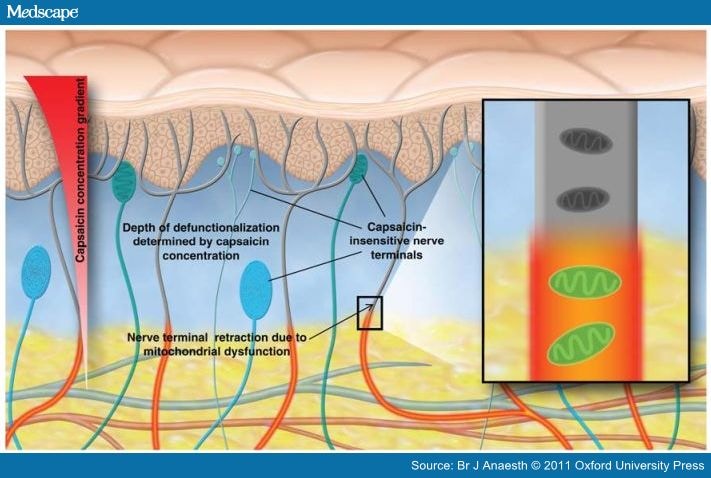
Pharmacological desensitization of TRPV1 itself may indeed contribute acutely to analgesic efficacy. However, transient effects on TRPV1 are quite unlikely to account for the persistent pain relief seen clinically after either single treatments with high-concentration capsaicin or repetitive administration of low-concentration capsaicin. Hence, the emerging preferred term for the persistent local effects of capsaicin is 'defunctionalization', which avoids conceptual confusion with the intrinsic desensitisation of the TRPV1 receptor.
Loss of mitochondrial function due to calcium overload and inhibition of metabolism may render affected nerve processes unable to maintain plasma membrane integrity and thus cause collapse of nerve endings to the depth where the capsaicin exposure was insufficient to irreversibly overwhelm mitochondrial function. If nerve fibres in skin retract or 'degenerate' to the depth at which mitochondrial function was preserved, it is expected that markers for any constituent of those fibres will show reductions.
Topical Capsaicin for Pain Management,2011
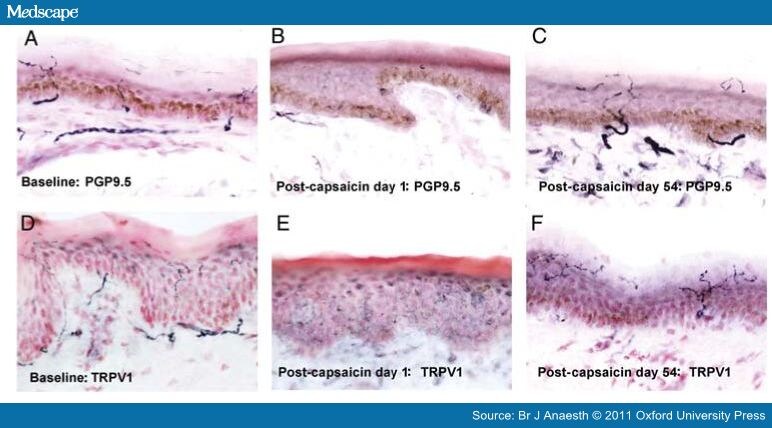
Topical capsaicin treatment leads to a reversible loss of ENFs. Human leg (calf) skin biopsies.
SUBSTANCE P
A persistent confusion which continues to appear in the medical literature involves the role of 'substance P depletion' in capsaicin-induced pain relief.
In the early and mid-1980s, researchers observed that skin substance P levels were also significantly reduced after topical treatment with capsaicin. At that time, substance P was thought to be a fundamentally important signal for pain neurotransmission, however, since then, substance P receptor antagonists have failed as analgesics in a number of clinical trials, and it is now widely recognized that of all the neuropeptides released by C-fibres, substance P is a more likely potential contributor to pain pathophysiology.
If nociceptive nerve fibres retract from the epidermis and dermis, then all markers they contain will be lost, and substance P is just one of many. The reduction of substance P content in skin after topical capsaicin administration is thus consequent to this process of nerve fibre defunctionalization and retraction.
NK1 (substance P) receptor antagonists--why are they not analgesic in humans?,2000
CAPSAICIN MECHANISM AS ANALGESIC DRUG
Capsaicin is a very lipophilic, non-water-soluble compound and resists diffusion into aqueous solutions such as blood, and shows limited potential for transdermal delivery across human skin. Even when capsaicin is absorbed systemically, the duration of exposure is very short.
Capsaicin is metabolized rapidly by several cytochrome (CYP) enzymes present in the human liver, but in vitro studies show that its metabolism in human skin is quite slow.
Some of the defunctionalization mechanisms discussed above can occur very rapidly and in vitro loss of capsaicin responsiveness may develop within 20 s. By driving cutaneous nociceptors to a defunctionalized state quickly, the inevitable burning sensation may be greatly reduced. Indeed, in clinical studies with capsaicin 8% patch, <2% of patients asked for early removal of the patch due to intolerance.
The preferential target for topically administered capsaicin appears to be cutaneous nociceptors. Defunctionalization of these nociceptors would be expected to produce pain relief if they are spontaneously active or hypersensitive, or help maintain pain syndromes by retrograde transport of excitatory trophic factors which contribute to neuronal hyperexcitability( mediated by reduced receptor thresholds, spontaneous discharges, and exaggerated responses to stimulation).
Indeed, evidence exists of a role for both these mechanisms in painful peripheral neuropathies and some other chronic pain syndromes.
After peripheral nerve lesions in primate and rodent models, spontaneous activity (with an incidence up to ~50%) develops in uninjured nociceptors that share the same innervation territory of the transected fibres.
This happens also in many pathologies, when some fibres get damaged, including Post Herpetic Neuralgy, painful diabetic neuropathy (PDN), painful HIV-associated neuropathy (HIV-AN), complex regional pain syndrome,metabolic syndrome (diabetic foot ulcers), and Fabry disease.
As previously discussed, the lower the density of cutaneous nociceptors, the more likely those nociceptors are to be hyperactive and the more readily they may respond to topical capsaicin.
In vitro hepatic and skin metabolism of capsaicin,2008
CAPSAICIN SIDE EFFECTS AND SAFETY
Capsaicin has been widely consumed orally by humans throughout the world over centuries and comprehensive reviews of its safety have not identified serious toxicity, although the presumed lack of toxicity of capsaicin in food does not preclude adverse effects related to its actions on the skin, topical capsaicin is also generally regarded as safe, either for medical or for cosmetic uses. The primary adverse effects seem to be local, transient, application site reactions, mainly pain and erythema.
The theoretical concern most relevant to chronic pain management is that in peripheral neuropathies associated with cutaneous denervation, defunctionalization of cutaneous nociceptors could leave areas of skin without sufficient protective sensation to prevent or avoid injury. In addition, cutaneous C-fibres may play a role in blood flow regulation and wound repair, as the substance P and neurokinin A released by C-fibres can stimulate growth of fibroblasts and epithelial cells.
However, these theoretical effects have not been observed in clinical practice.
Topical capsaicin. The fire of a 'hot' medicine is reignited,2010
In six large double-blind trials with comparators and in other smaller studies with no reports relating to either loss of protective sensations or impaired wound healing. In an experimental model which uses a 48 h application of 0.1% w/w capsaicin cream under an occlusive patch to induce nearly complete loss of immunostaining for ENFs and a very robust effect on dermal nociceptors, no effect on wound healing has been reported in PDN and HIV-AN patients after repeated 3 mm punch biopsies.
CONCLUSIONS
In conclusion, directed TRPV1 agonist therapies, in which nociceptor defunctionalization is restricted to discrete target organs such as the skin, may be an attractive treatment to control localized pain or hypersensitivity.
There is good evidence for hyperactivity of cutaneous nociceptive C-fibres in numerous pain syndromes, and thus topical TRPV1 receptor agonist-mediated defunctionalisation warrants evaluation as an approach for their management. Although the 'substance P depletion' hypothesis was attractive in the 1980s, subsequent advances in our understanding of cutaneous innervation and the responses to topical capsaicin have rendered this hypothesis less relevant. Reduction of substance P content in the skin is just one of many consequences of defunctionalisation.
An important advantage of the topical capsaicin approach is that this drug is poorly absorbed transdermally in humans and there appear to be few systemic adverse effects or even local effects other than transient application-site reactions such as pain and erythema. The recent approval of the capsaicin 8% patch, which is designed to be used episodically, provides an alternative to low-concentration capsaicin-based medicines by providing for longer term pain relief in some patients, while avoiding the requirement for repeated daily self-administration, lack of patient compliance, and possible home environmental contamination. Given the common use of topical capsaicin in a wide variety of chronic pain syndromes and other conditions, we look forward to further clinical evaluations of capsaicin 8% patch and other innovative topical capsaicin formulations.
Topical capsaicin for chronic neuropathic pain in adults,2009