Serra Enrica, Crosio Alessandro
DESCRIPTION

Qlaira (Klaira IT) is a four-phasic estradiol valerate (E2V)/dienogest (DNG) combined oral contraceptive pill.
Discovery of the dose-dependent risks of adverse hormonal events induced the development of low-dose ethinylestradiol and/or novel progestin combinations.
Until recently, the primary estrogen utilized in Combined Oral Contraception (COC) was ethinylestradiol. Utilization of the natural estrogen estradiol in COC was investigated as estradiol was postulated to induce fewer thrombogenic effects. However, it was associated with poor cycle control. In order to combat the poor cycle control observed with earlier estradiol-containing COC, estradiol has been combined with a novel 19-nortestosterone derivative, dienogest, in a four-phasic formulation with an estrogen step-down and a progestogen step-up sequence.
Qlaira is composed by:
By providing early estrogen dominance, the dosing strategy is thought to allow for initial endometrial proliferation and sensitivity to progestogen action to develop, followed by the potent effects of DNG during the middle and late part of the cycle to ensure endometrial stroma stability and bleeding control.
Estradiol valerate is the esterified form of 17βestradiol (E2) with valeric acid, and is hydrolysed to estradiol soon after oral administration. E2 is the most potent of the naturally occurring estrogens and is the natural estrogen produced by the ovary. 1mg of estradiol valerate corresponds to 0.76mg of E2 and to 10μg of EthinylEstradiol (EE).

Dienogest is a progestogen, similar to 19-nortestosterone derivatives, with 17α-cyano- methyl group. It combines the properties of both progesterone and 19-nortestosterone derivatives and has a strong progestational effect on the endometrium. It has a relatively low inhibition of gonadotropin secretion (infact doses are in milligram range) and anti-androgenic activity.
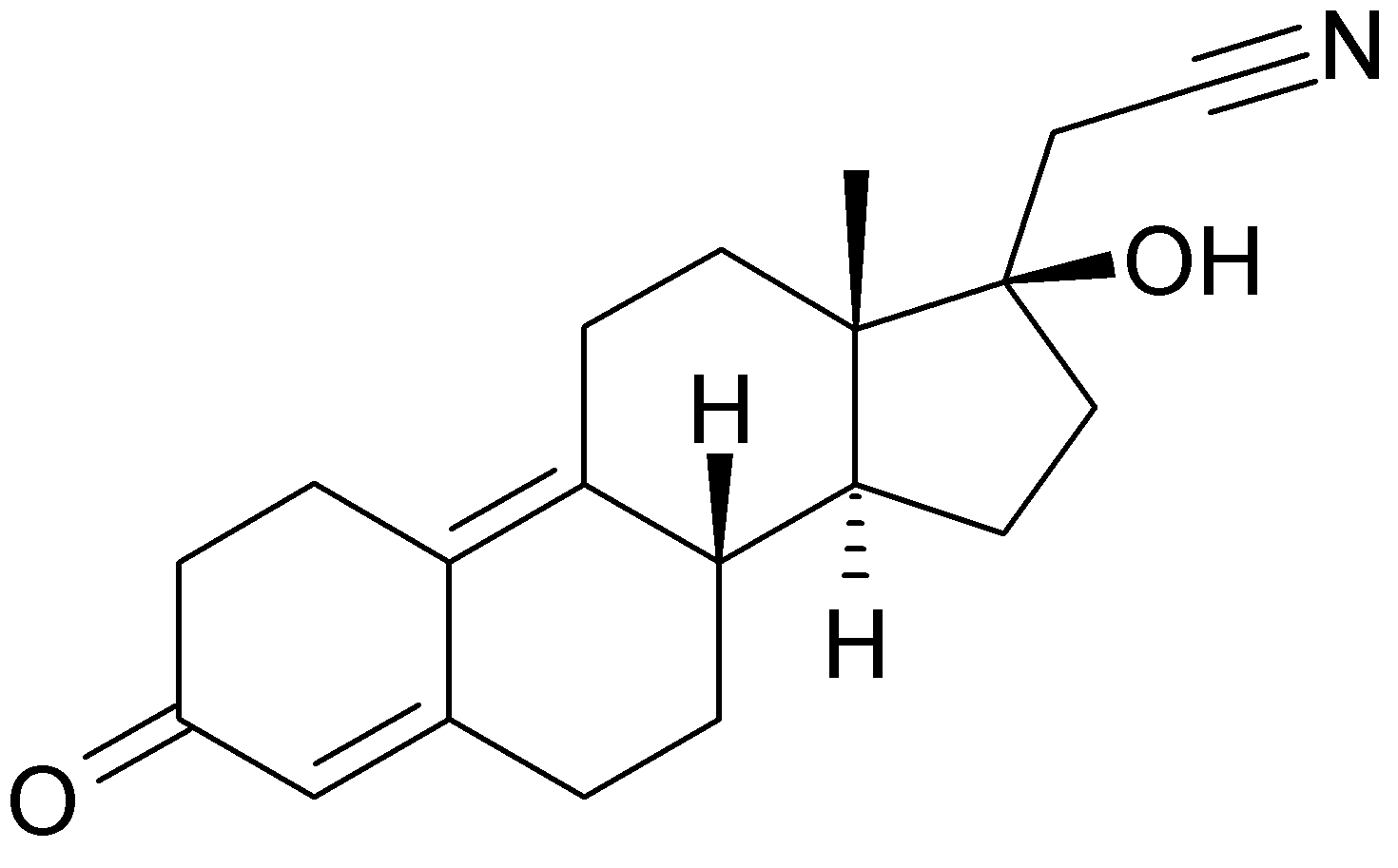
CLASSIFICATION
The Combined Oral Contraceptive Pill is classificated in function of the change in ratio of the estrogenic and progestin during the 28 days assumption in:
- Monophasic: 21 tablets of estrogen and progestin in constant amount, followed by 7 days suspension or 7 tablets of placebo or else an iron supplement.
- Multiphasic: bi, tri, four phasic if the dosage of one or both components is varied one, two or three times during the cycle of active tablets.
INDICATIONS
Qlaira is used to prevent pregnancy in all women, especially those who present menorrhagia. Is also indicated as simptomatic treatment in Policystic Ovary Sindrome and Endometriosis.
This pill should not be a first-line product for most healthy new starts women because of there are many less expensive, highly effective and well-tolerated generic oral contraceptive products on the market. Moreover the missed pill rules may make compliance more difficult for less experienced users. At the same time, the multiple proven benefits and theoretical advantages of estradiol make this an important new choice for many women, particularly those with heavy menstrual bleeding or those who simply desire shorter and lighter periods.
Estradiol valerate/dienogest is contraindicated in women with an acquired or hereditary predisposition for arterial or venous thrombosis, a history of or current arterial thrombosis, cerebrovascular accident, liver cancer, pancreatitis (if associated with severe hypertriglyceridaemia), severe hepatic diseases, a history of migraine with focal neurological symptoms, known or suspected sex-steroid influenced cancer, the presence of severe or multiple arterial or venous thrombosis risk factors, including diabetes mellitus with vascular symptoms, severe dislipoproteinaemia and severe hypertension or undiagnosed vaginal bleeding. This controindications arise from the biochemical effects of estrogen especcialy on liver metabolism.
The tablets should be taken every day at the same hour, following the order in blister. The next package should be started on the day after the last assumption, gapless. The menstruation appears generally during assumption of the last two tablets of 28 days (placebo) and usually does not stop during first tablets of new package, but within first days of new treatment.
PHARMACOKINETICS
- Estradiol Valerate
- Absorption: after oral administration, Estradiol valerate is completely absorbed by the intestinal mucosa. Following initial absorption, E2V is hydrolyzed to yield E2 and valeric acid by metabolic process that occours in the gastrointestinal mucosa and the liver.
- Metabolism: Estradiol metabolism occurs in the gastrointestinal mucosa and liver. Estradiol valerate undergoes extensive first-pass metabolism, giving rise to estradiol and its metabolites serum concentration. That proceses result in the metabolism of approximately 95% of the oral estradiol valerate dose prior to its entry into the systemic circulation, so that the bioavailability of E2V is about 3-5%. Metabolism of E2 in intestinal mucosa and liver leads to conversion to estrone (E1) and estriol (E3). Both E1 and E3 are metabolized to their sulfate and glucuronide forms; these forms have limited cell penetration, partially explaining their lower potency. The estrone (E1) is converted to estrone sulfate (E1-S) by estrogen sulfotransefare and both can be converted back to E2, so E1-S is thought to be a circulating storage form of the hormone. Since most of the orally absorbed E2 is converted to E1 and E1-S, these stabilize the serum levels of E2 achieved with daily dosing. Estrogens undergo further oxidation through the hepatic cytochrome P450 pathway CYP3A4 and 1A2 primarly before elimination.
- Distribution: 5% of oral dose of E2V reach the sistemic circulatrion as E2. E2 is bound 38% to sex hormone binding globulin (SHBG), 60% to albumin, and 2% to 3% circulates in a free form. The apparent volume of distribution is ∼1.2 L/kg. The serum E2 concentration remains stable during the 28-days treatment period in range 0.0336–0.0647 ng/mL due to steady state between E2⇔E1 ⇔ E1-S and entero-epatich recirculation. The recirculation involves primarly E1-S and Estrone glucuronide. The plasma half-life of estradiol is 1.5 hours, but the terminal elimination half-life of estradiol following oral administration is 13–20 hours and is dependent upon entero- hepatic recirculation and circulating estrogen sulfate and glucuronide levels.
- Excrection: Estradiol and its metabolites are predominately excreted in the urine. Minimal (10%) excretion occurs via the faeces.
- Dienogest
- Absorption: DNG is almost completely absorbed following oral administration and has high bioavailability (91%). No clinically rilevant effect on the rate and extent of DNG absorption is observed with concomitant food admnistration.
- Distribution: DNG has a distribution volume of 46L. It circulates in serum primarly bound to serum albumin (90%) yielding 9% free and bioavailable. This high percentage of bioavailable hormone it thought to contribute to the potyent endometrial effects. DNG han no specific affinity for SHBG or Cortisol Binding Globuline and does not displace testosteron from SHBG or increase bioavailable testosteron. Serum concentration of DNG in steady state are: min 11.8ng/mL; max 82.9ng/mL; med 33.7ng/mL.
- Metabolism: DNG’s plasma half-life is about 12h. DNG is almost completely metabolized by cytochrome P450, isoform CYP3A4 in pharmacologically inactive metabolites. The unchanged drug constituting »50% of the circulating dienogest compounds in the plasma.
- Excrection: DNG and his metabolites are secreted mainly by the kidneys. 86% of the hormon and its metabolites are eliminated in 6 days.
MOLECULAR MECHANISM
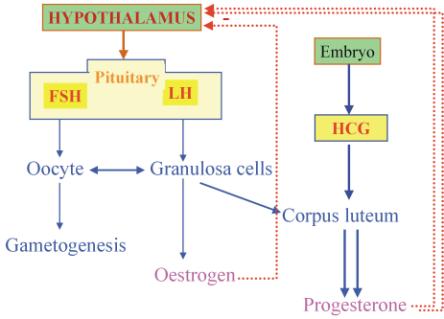
The combinations of estrogens and progestins exert their contraceptive effect largely through selective inhibition of pituitary function that results in inhibition of ovulation. The combination agents also produce a change in the cervical mucus, in the uterine endometrium, and in motility and secretion in the uterine tubes, all of which decrease the likelihood of conception and implantation.
Hypothalamic actions of steroids play a major role in the mechanism of oral contraceptive action.
Progestational effects include:
- Inhibition of ovulation by suppressing luteinizing hormone (LH) by reduction frequency of GnRH pulses throught natural negative feedback mechanism that occours during luteal phase of ovary cicle. Since the proper frequency of LH pulses is essential for ovulation, this effect of progesterone likely plays a major role in the contraceptive action of these agents. The progestin component may also inhibit the estrogen-induced LH surge at mid-cycle.
- Thickening of cervical mucus, thus hampering the transport of sperm;
- Possible inhibition of sperm capacitation;
- Hampered implantation by the production of decidualized endometrium with exhausted and atrophic glands, endometrium that is not receptive to implantation.
Estrogenic effects include:
- Partial inhibition of ovulation in part by the suppression of follicle-stimulating hormone (FSH) during the follicular phase, contributing to lack of follicular development, and luteinizing hormone (LH).
- Alteration of secretions and cellular structures of the endometrium.
Molecular mechanism of Estrogens
Estrogens exert their effects via estrogen receptors, which are located throughout the female reproductive tract, particularly the ovaries, uterus and vagina, and in bone, brain, endothelial cells, hypothalamus, lungs, mammary glands and vascular smooth muscle.
The balance between estrogen and progestin effects influences the bleeding profile of a combination OC. Estrogen induces endometrial proliferation. Progestogens oppose the mitotic action of estrogen, leading to a stable decidualized endometrium. Progestogen withdrawal during the hormone-free interval stimulates a series of signals that irreversibly lead to endometrial shedding within 48 h.
Molecular mechanism of progesterone:
Dienogest act throught the progesterone receptor, but has demonstrated limited binding affinity (approximately 10% relative to progesterone) to this protein in human uterine tissue; however, despite this, dienogest has exhibited a strong progestogenic effect in vivo. Dienogest has negligible binding affinities for the estrogen, glucocorticoid and mineralocorticoid receptors and a low binding affinity for the androgen receptor in vitro; it has exhibited antiandrogenic activity in vitro and in vivo.
There is a single gene that encodes two isoforms of the progesterone receptor (PR): PR-A and PR-B. The ratios of the individual isoforms vary in reproductive tissues as a consequence of tissue type, developmental status, and hormone levels. Both PR-A and PR-B have AF-1 and AF-2 transactivation domains, but the longer PR-B also contains an additional AF-3 that contributes to its cell- and promoter-specific activity. Since the ligand-binding domains of the two PR isoforms are identical, there is no difference in ligand binding. In the absence of ligand, PR is present in the nucleus in an inactive monomeric state bound to heat-shock proteins (HSP-90, HSP-70, and p59).
Upon binding progesterone or analogues, the heat-shock proteins dissociate, and the receptors are phosphorylated and subsequently form dimers (homo- and heterodimers) that bind with high selectivity to PREs (progesterone response elements) located on target genes. Transcriptional activation by PR occurs primarily via recruitment of co-activators such as SRC-1, NcoA-1, or NcoA-2. The receptor-co-activator complex then favors further interactions with additional proteins such as CBP and p300, which have histone acetylase activity.
Histone acetylation causes a remodeling of chromatin that increases the accessibility of general transcriptional proteins, including RNA polymerase II, to the target promoter.
The biological activities of PR-A and PR-B are distinct and depend on the target gene in question. In most cells, PR-B mediates the stimulatory activities of progesterone; PR-A strongly inhibits this action of PR-B and is also a transcriptional inhibitor of other steroid receptors.
Certain effects of progesterone, such as increased Ca2+ mobilization in sperm, can be seen in as little as 3 minutes, and these effects are caused by nongenomic mechanisms involving membrane-bound progesterone receptors that are not derived from the gene encoding PR-A/PR-B.
The presence of Estrogen and Progesterone receptors in many tissues leads to changes during COC assumption:
- Effects on the ovary: Chronic use of COC depresses ovarian function. The great majority of patients return to normal menstrual patterns when these drugs are discontinued.
- Effects on the uterus: assumption of estrogens and progestins produce further morphologic and biochemical changes of the endometrial stroma under the influence of the progestin, which also stimulates glandular secretion throughout the luteal phase. After prolonged use, the cervix may show some hypertrophy and polyp formation. There are also important effects on the cervical mucus, making it thicker and less copious.
- Effects on breast: Stimulation of the breasts occurs in most patients receiving estrogen-containing agents. Some enlargement is generally noted.
- Effects on the central nervous system: estrogens tend to increase excitability in the brain, whereas progesterone tends to decrease it. Although the incidence of pronounced changes in mood, affect, and behavior appears to be low, milder changes are commonly reported.
- Effects on endocrine function: Estrogens given orally increase the plasma concentration of the a2 globulin that binds cortisol (corticosteroid-binding globulin).
E2V/DNG cause alterations in the renin-angiotensin-aldosterone system. Plasma renin activity has been found to increase, and there is an increase in aldosterone secretion.
Thyroxine-binding globulin is increased. As a result, total plasma thyroxine (T4) levels are increased to those commonly seen during pregnancy. Since more of the thyroxine is bound, the free thyroxine level in these patients is normal.
Estrogens also increase the plasma level of SHBG and decrease plasma levels of free androgens by increasing their binding; large amounts of estrogen may decrease androgens by gonadotropin suppression.
- Effects on blood: Serious thromboembolic phenomena could occurring in women taking oral contraceptives. The changes that have been observed are similar to those reported in pregnancy. There is an increase in factors II, VII, VIII, IX, and X and a decrease in antithrombin III and decrease the anticoagulation factors protein C, protein S, and antithrombin III. Fibrinolytic pathways also are affected with decreasing in levels of plasminogen-activator inhibitor protein-1 (PAI-1) and concomitant increasing in fibrinolysis. Thus, estrogens increase both coagulation and fibrinolytic pathways.
There is an increase in serum iron and total iron-binding capacity similar to that reported in patients with hepatitis.
- Effects on the liver: These hormones also have profound effects on the function of the liver. The effects on serum proteins result from the effects of the estrogens on the synthesis of the various a2 globulins and fibrinogen.
Important alterations in hepatic drug excretion and metabolism also occur.
- Effects on lipid metabolism: Estrogens increase high-density lipoprotein (HDL) levels and decrease the levels of low-density lipoprotein (LDL) and Lipoproteine Lipase. Estrogens also increase biliary cholesterol secretion and decrease bile acid secretion, leading to increased saturation of bile with cholesterol that results in gallstone formation in some women receiving estrogens.

- Effects on carbohydrate metabolism: Progesterone increases the basal insulin level and the rise in insulin induced by carbohydrate ingestion. However, the changes in glucose tolerance are reversible on discontinuing medication.
- Effects on the cardiovascular system: These agents cause small increases in cardiac output associated with higher systolic and diastolic blood pressure and heart rate. The pressure returns to normal when treatment is terminated. Although the magnitude of the pressure change is small in most patients, it is marked in a few. It is important that blood pressure be followed in each patient.

- Estrogen actions on the vascular wall include induction of inducible NO synthase and increased production of NO and prostacyclin, all of which promote vasodilation.
- Effects on the skin: The oral contraceptives have been noted to increase pigmentation of the skin (chloasma). Since ovarian androgen is suppressed, many patients note decreased sebum production, acne, and terminal hair growth.
COMPARISON BETWEEN EE/LNG and E2V/DNG
Ethinil Estradiol (EE) is the primary estrogen utilized in oral contraception. EE is a potent inducer of hepatic enzymes and globulins, and this induction continues even after the initial first pass following oral administration. By contrast, while oral administration of E2 (as E2V) stimulates the liver during initial first pass, the effect does not continue as physiologic levels are achieved, so the overall impact is lower than with a biologically equivalent dose of EE.
Although E2 has lower metabolic effects than EE, the current level of data from clinical trials of the E2V/DNG combination does not support any claim of improved safety over existing pills. Therefore, the current contraindications and cautions concerning OC prescription remain in place for this new formulation. . Additional evaluation of safety will require epidemiological studies planned as part of the Phase IV program for this product.
At present there are many studies that try to compare metabolic and sistemic effect of Qlaira to association of EE and Levonorgestrel (EE/LNG), a product considered to be the gold standard for safety:
- Cycle control included the incidence and characteristics (length, intensity and onset) of scheduled (withdrawal) and unscheduled (intracyclic) bleeding. Scheduled withdrawal bleeds were significantly shorter and lighter in women treated with E2V/DNG than in those treated with EE/LNG. The maximum intensity of scheduled withdrawal bleeding over the seven cycles was also significantly lighter in women treated with E2V/DNG than in those receiving EE/LNG. More E2V/DNG users experienced amenorrhea (15.4% of cycles with E2V/DNG and 4.5% of cycles with EE/LNG.). Intracyclic (unscheduled) bleeding occurred in approximately 14% of women treated with E2V/DNG and in 12% of women treated with EE/LNG, with the proportions similar during all cycles and the highest rate occurring in the first cycle of treatment in both groups. Intensity of unscheduled bleeding was similar between groups.
- Adverse events: The adverse events reported from clinical trials with E2V/DNG are typical of other recently approved low-dose oral contraceptives.
- Haemostatic parameters: E2V/DNG and EE/LNG appear to exert no clinically relevant effects on the majority of haemostatic parameters, with values predominately remaining within the normal reference ranges (study in 29 women); this studies reveald no significant differences in levels of prothrombin fragment (indicative of thrombin turnover), factors VII (VIIc) and VIII (VIIIc), anti-thrombin III, proteins C and S, plasminogen activator inhibitor (PAI)-1 activity, and activated protein C resistance. Instead, prothrombin was higher than the normal range and PAI-1 antigen was lower than the normal range in both groups. Also mean absolute D-dimer levels (indicative of fibrin turnover) remained within the normal range in the E2V/DNG and EE/LNG groups; however, the mean intraindividual change from baseline in D-dimer levels was significantly greater in the EE/LNG group (mean absolute level after three cycles 353 ng/mL) than in the E2V/DNG group (mean absolute level after three cycles 237 ng/mL): increased by 39 (E2V/DNG) vs 158 (EE/LNG) ng/mL.
- Plasma proteins: Sex hormone binding globulin (SHBG) levels were elevated from baseline by 63% following E2V/DNG and by 112% following EE/LNG therapy (mean levels exceeding the normal range). Cortisol binding globulin (CBG) levels were elevated from baseline by 28% following E2V/DNG and and 146% following EE/LNG therapy; mean values for both treatment groups were within the normal range.
- Lipid metabolism: no significant between-group differences were observed in the mean intraindividual relative changes from baseline in plasma HDL, and LDL cholesterol levels in E2V/DNG and EE/LNG therapy. Women in both treatment groups had increases from baseline in triglyceride (31.4% in the E2V/DNG group vs 32.0% in the EE/LNG group) and VLDL cholesterol (27.3% vs 48.5%) levels.
Efficacy
Data regarding the contraceptive efficacy of E2V/DNG has been derived from three large-scale pivotal trials conducted in Europe and North America [1] [2] [Efficacy of estradiol valerate/dienogest OC: results of 3 large studies in North America and Europe]. The efficacy (Pearl Index, PI: number of pregnancies per 100 women years of exposure) and safety (adverse events) included in the European SPC (summary of product characteristics) is based on results from these three pivotal studies.
Taken together, a total of 2266 women received E2V/DNGin the three trials, resulting in a total treatment exposureof 2513 women years. A total of 16 pregnancies occurred either during cycles 1 -- 13 or within 14 days after the end of treatment (PI 1.27; upper limit of 95% CI 2.06). Removing the nine pregnancies attributed to user failure (e.g., non-use or incorrect use of the method) yielded an adjusted first-year PI of 0.72 (upper limit of 95% CI 1.37).
The largest trial was a multicenter, open-label, noncomparative, 20-cycle study conducted in 50 centers in Europe [2]. In the full analysis set, 13 pregnancies occurred during an exposure time of 23,368 cycles (PI of 0.73; upper limit of 95% CI 1.24), six of which were attributed to method failure (adjusted PI 0.34; upper limit of 95% CI 0.73). The length (median 4 days) and intensity (median score of light) of withdrawal bleeding tended to decrease over the treatment cycles.
SIDE EFFECTS
Side effects were recorded in 3 phase III clinical studies (N=2,266 women at risk for pregnancy, see above).
The three most frequently reported adverse drug reactions were breast discomfort and pain (4.9%), metrorrhagia (4.9%) and headache (3.1%). Other treatment emergent adverse events occurring in +1% of women included acne (2.8% of women), increase in bodyweight (1.5%), amenorrhoea (1.7%), dysmenorrhoea (1.7%) and abdominal pain (1.7%).
Other side effects considered at least possibly causally related to Qlaira use were:
- Metabolism and nutrition disorders: increased appetite (0.1-1%), fluid retention and hypertriglyceridaemia (<0.1%).
- Psychiatric disorders: depression/depressed mood, libido decreased, mental disorder, mood change (0.1-1%), affect lability, aggression, anxiety, dysphoria, sleep disorder and stress (<0.1%).
- Nervous system disorders: dizziness (0.1-1%), disturbance in attention, paraesthesia, and vertigo (<0.1%).
- Vascular disorders: hypertension (0.1-1%), bleeding varicose vein and vein pain (<0.1%).
- Hepatobiliary disorders: alanine aminotransferase increased, focal nodular hyperplasia of the liver (<0.1%).
- Skin and subcutaneous tissue disorders: alopecia, pruritus, rash (0,1-1%), allergic skin reaction, chloasma, dermatitis, hirsutism, pigmentation disorder and seborrhoea (<0.1%).
- Musculoskeletal and connective tissue disorders: back pain, muscle spasms and sensation of heaviness (<0.1%).
- Reproductive system and breast disorders: dyspareunia, fibrocystic breast disease, menstrual disorder, ovarian cyst, pelvic pain, premenstrual syndrome, uterine spasm, breast enlargement (0.1-1%), coital bleeding, genital hemorrhage, vaginal burning sensation, galactorrhea, breast cyst and benign breast neoplasm (<0.1%).
- Serious adverse events considered to be at least possibly related to study medication occurred in 0.3% of women, and included cases of a ruptured ovarian cyst and deep vein thrombosis, myocardial infarction, focal nodular hyperplasia of the liver, ocular histoplasmosis, breast mass, uterine leiomyomata and cervical dysplasia.
Venous thromboembolic disease: the overall incidence of these disorders in patients taking low-dose oral contraceptives is about threefold higher than women not taking oral contraceptives. Until additional evaluation of safety won’t be available, the thromboelmolic risk must be considered also using Qlaira such as other COCs. The risk for this disorder is increased during the first month of contraceptive use and remains constant for several years or more. The risk returns to normal within a month when use is discontinued. The risk is increase in women who presents predisposing conditions to venous thrombosis such as stasis, altered clotting factors such as antithrombin III, increased levels of homocysteine, or injury, genetic disorders, including mutations in the genes governing the production of protein C, protein S.
Estrogen is the component resposeable of increased risk and the major plasma inhibitor of thrombin, antithrombin III, is substantially decreased during oral contraceptive use.
Cancer: The occurrence of malignant tumors in patients taking oral contraceptives has been studied extensively. It is now clear that these compounds reduce the risk of endometrial and ovarian cancer. The lifetime risk of breast cancer in the population as a whole does not seem to be affected by oral contraceptive use. A meta-analysis from 54 epidemiological studies reported that there is a slightly increased relative risk (RR = 1.24) of having breast cancer diagnosed in women who are currently using COCs. The excess risk gradually disappears during the course of the 10 years after cessation of COC use. Because breast cancer is rare in women under 40 years of age, the excess number of breast cancer diagnoses in current and recent COC users is small in relation to the overall risk of breast cancer. These studies do not provide evidence for causation. The observed pattern of increased risk may be due to an earlier diagnosis of breast cancer in COC users, the biological effects of COCs or a combination of both. The breast cancers diagnosed in ever-users tend to be less advanced clinically than the cancers diagnosed in never-users. The relation of risk of cervical cancer to oral contraceptive use is still controversial. An increased risk of cervical cancer in long-term users of COCs (> 5 years) has been reported in some epidemiological studies, but there continues to be controversy about the extent to which this finding is attributable to the confounding effects of sexual behaviour and other factors such as human papilloma virus (HPV). It should be noted that a number of recent studies associate the use of oral contraceptives by women with cervical infection with the human papillomavirus to an increased risk of cervical cancer. In rare cases, benign liver tumours, and even more rarely, malignant liver tumours have been reported in users of COCs. Then women wishing to use combined OC can be reassured that their decision is unlikely to place them at higher risk of developing cancer.
Overdose: There have been no reports of serious deleterious effects from overdose. Symptoms that may occur in case of taking an overdose of active tablets are: nausea, vomiting and, in young girls, slight vaginal bleeding. There are no antidotes and further treatment should be symptomatic.
INTERACTIONS
Interactions of other medicinal products on Qlaira:
The metabolism of both components (E2V/DNG) is prerogative of cytochrome P450 isoform 3A4 mainly, so the circulating levels and the efficacy of preparation depends on the contemporary presence of other molecules that can inhibit or stimulates the enzymatic complex.
Phenobarbital, barbiturates, primidone, carbamazepine, phenytoin, rifampicina and possibly oxcarbazepine, topiramate, felbamate, griseofulvin and the herbal remedy St. John’s wort (hypericum perforatum) may reduce plasma concentrations of estrogens, possibly resulting in a decrease in therapeutic effects and/or changes in the uterine bleeding profile. The mechanism of this interaction appears to be based on the hepatic enzyme-inducing properties (CYP 3A4 enzymes) of these drugs which can result in increased clearance of sex hormones. Maximal enzyme induction is generally not seen for 2-3 weeks but may then be sustained for at least 4 weeks after the cessation of drug therapy. Women should be counseled to always use a secondary or backup method of contraception when enzyme inducers are used. In women on chronic treatment with hepatic enzyme-inducing active substances, another reliable, non-hormonal, method of contraception is recommended.
Serum hormone levels are elevated with the use of strong CYP 3A4 enzyme inhibitors such as ketoconazole, itraconazole, erythromycin, clarithromycina, ritonavir, cimetidine, verapamil, macrolides, diltiazem, antidepressants and grapefruit juice may increase plasma concentrations of estrogens and dienogest and may result in side effects.
The concomitant administration with HIV protease inhibitors has resulted in either increases or decreases of plasma levels of estradiol and dienogest.
Although clinical studies have not proved that antibiotics have consistent effects on serum concentrations of these hormones, pregnancies have been reported. Antibiotics reduce intestinal bacterial flora that’s necessary to entero-hepatic recirulation of estrogens. Entero-hepatic recirculation is necessary to preserve stable serum levels of estrogens and exert the inibition of ovulation. Intestinal bacteria cleave the binding between glucuronic acid and estrogen, so that the hormones can be absorbed by enterocites.
Interactions of Qlaira on other medicinal products:
Qlaira can decrease the plasma concentration of lamotrigine via glucuronidation. As a result, dosage adjustments of lamotrigine may be required.
Laboratory tests:
The use of contraceptive steroids may influence the results of certain laboratory tests, including biochemical parameters of liver, thyroid, adrenal and renal function, plasma levels of (carrier) proteins, e.g. corticosteroid binding globulin and lipid/lipoprotein fractions, parameters of carbohydrate metabolism and parameters of coagulation and fibrinolysis. Changes generally remain within the normal laboratory range.
BIBLIOGRAPHY
- "Evaluation of a new estradiol oral contraceptive: estradiol valerate and dienogest"; Jeffrey T Jensen (2010)
- "Review of the safety, efficacy and patient acceptability of the combined dienogest/estradiol valerate contraceptive pill"; Maurizio Guida, Giuseppe Bifulco, Attilio Di Spiezio Sardo, Mariamaddalena Scala, Loredana Maria Sosa Fernandez, Carmine Nappi (August 2010)
- "Estradiolo Valerate and Estradiolo Valerate/Dienogest (Natazia) Tablets: The first four-phasic oral contraceptive"; Uche Anadu Ndefo, Nina Mosely; (2010).
- "Estradiol Valerate/Dienogest In Oral Contraception"; Sheridan M. Hoy and Lesley J. Scott (2009).
- "Ovulation inhibition with four variations of a four-phasic estradiol valerate/dienogest combined oral contraceptive: results of two prospective, randomized, open-label studies"; Jan Endrikat, Susanne Parke, Dietmar Trummer, Werner Schmidt, Ingrid Duijkers, Christine Klipping; (2008).
- CONFERENZA STAMPA "DOPO 50 ANNI LA CONTRACCEZIONE DIVENTA “BIO” ARRIVA IN ITALIA KLAIRA, LA PRIMA PILLOLA NATURALE" 8° Congresso della European Society of Gynecology, Roma, Centro Congressi Santo Spirito in Saxia, 10 settembre 2009.
- "Basic and Clinical Pharmacology"; 10th Edition; Bertram Katzung, Anthony Trevor, Susan Masters.
- "Goodman & Gilman - Le basi farmacologiche della terapia" 11/ed; Laurence L. Brunton, John S. Lazo, Keith L. Parker.