The Thyroid hormones
The thyroid gland is
located around the trachea
and its blood flow is
sensitive to the
respiratory rate
The Hormone

.
.
.
.
The Synthesis
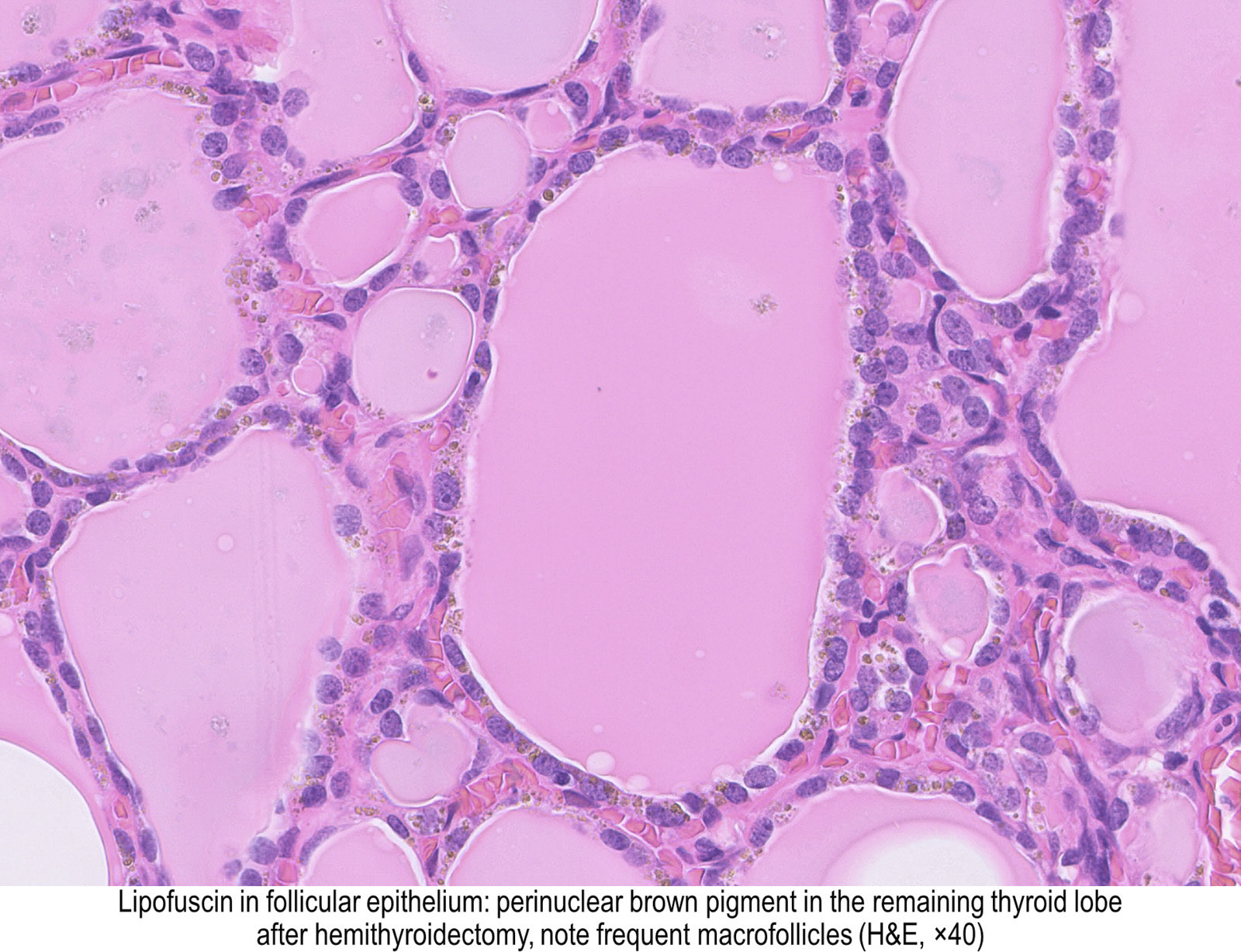
Thyroid follicles


Requirements:

Iodine uptake into the thyrocytes
Active transport of iodide into the thyroid gland is a crucial and rate-limiting step in the biosynthesis of thyroid hormones. It is mediated by a specific sodium-dependent iodide transporter located at the basolateral membrane of thyroid follicular cells, the sodium iodide symporter/NIS, C Spitzweg, JC. Morris, 2002.

Active transport of iodide into the thyroid gland is a crucial and rate-limiting step in the biosynthesis of thyroid hormones. It is mediated by a specific sodium-dependent iodide transporter located at the basolateral membrane of thyroid follicular cells, the sodium iodide symporter/NIS.
Pituitary-derived TSH had been known for decades to stimulate iodide transport into the thyroid gland via the adenylate cyclase cAMP pathway
The iodine metabolism according to L. Fugazzola - 

Redox up-regulated expression of rat liver manganese superoxide dismutase and Bcl-2 by thyroid hormone is associated with inhibitor of κB-α phosphorylation and nuclear factor-κB activation
Fernandez V;
JOURNAL OF ENDOCRINOLOGY
2005, Volume: 186(3) Page(s): 539-547
The Release
Role of thyroglobulin endocytic pathways in the control of thyroid hormone release 2000
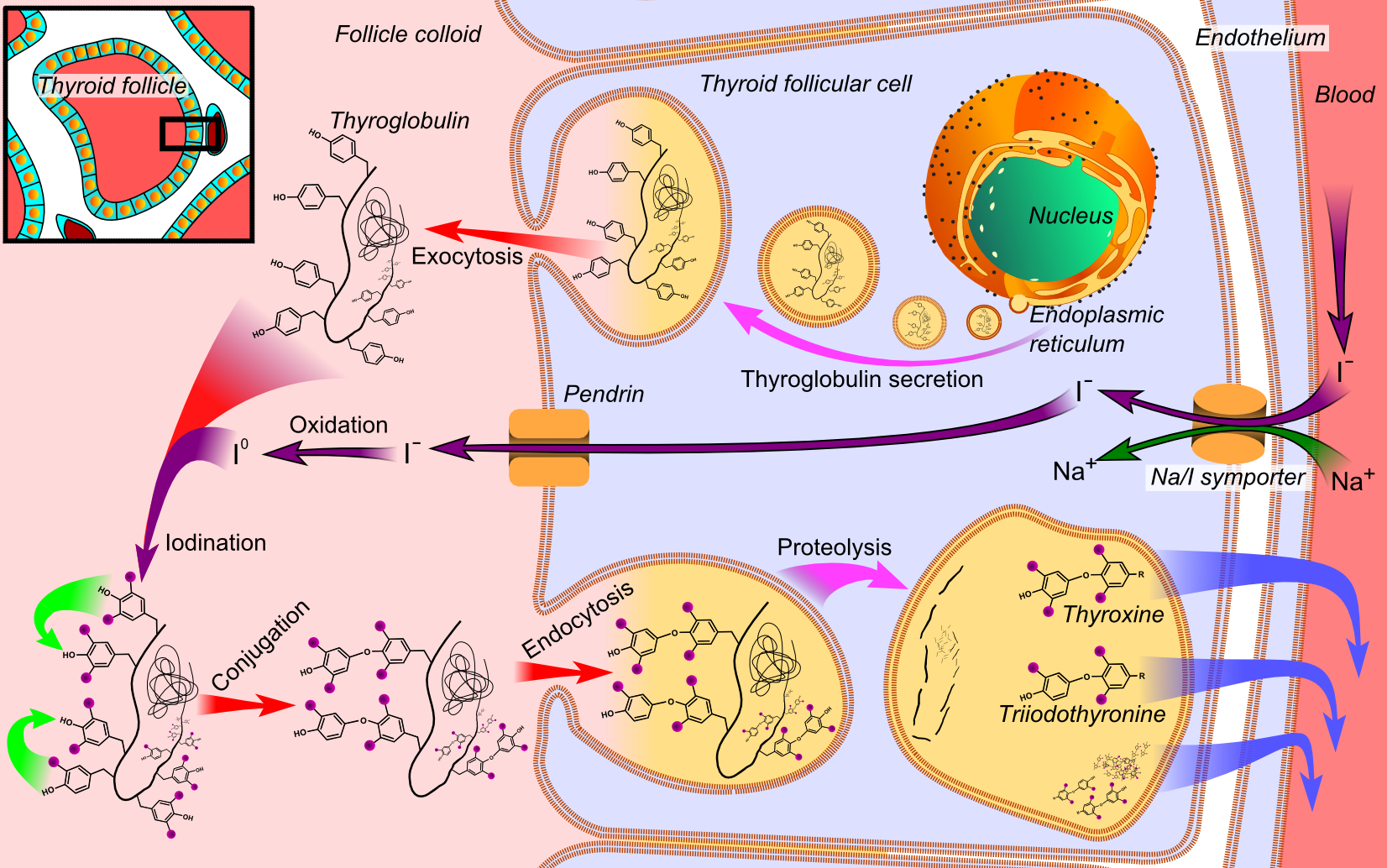
Megalin mediates Tg uptake by thyrocytes, especially under intense thyroid-stimulating hormone stimulation, resulting in transcytosis of Tg from the colloid to the bloodstream
The degradation
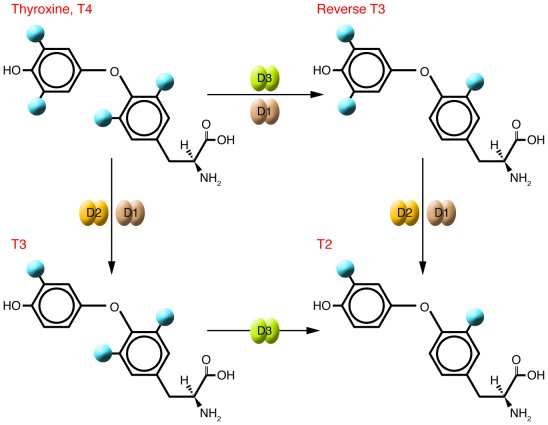
Iodotyrosine deiodinase (a member of the NADH oxidase/flavin reductase family) activity is regulated by NADH/NAD ratio
a deeper insight
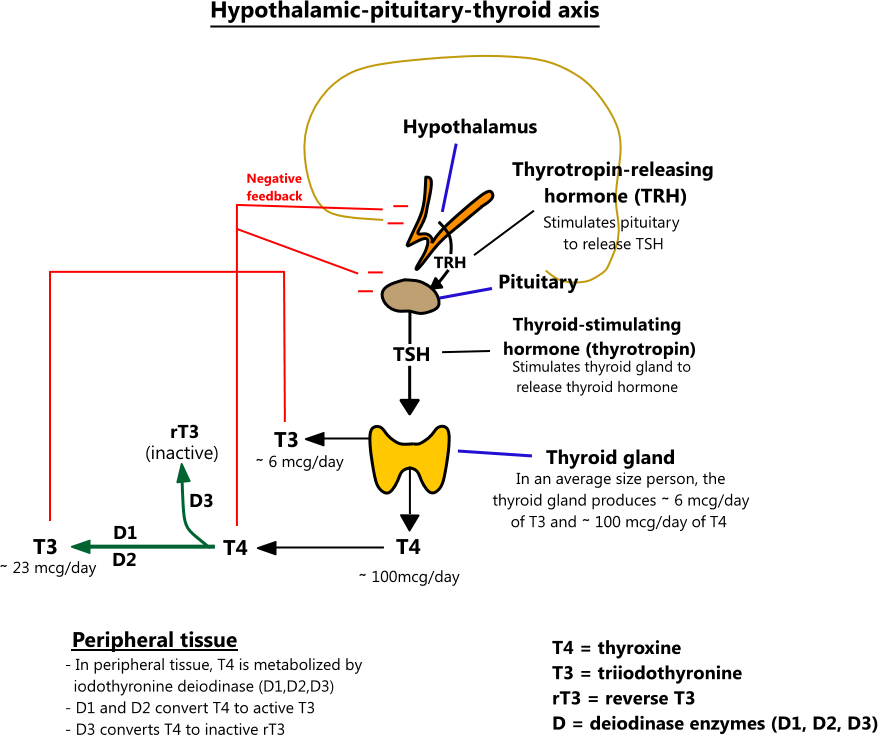

Drugs effecting TH function
The Circadian Rythm

TH Target Tissues
TH enter and are metabolized differently in different tissues

TH and the liver
Thyroid Hormone Signaling and the Liver, 2020
- Abstract
Thyroid hormone (TH) plays a critical role in maintaining metabolic homeostasis throughout life. It is well known that the liver and thyroid are intimately linked, with TH playing important roles in de novo lipogenesis, beta-oxidation (fatty acid oxidation), cholesterol metabolism, and carbohydrate metabolism. Indeed, patients with hypothyroidism have abnormal lipid panels with higher levels of low-density lipoprotein levels, triglycerides (triacylglycerol; TAG), and apolipoprotein B levels. Even in euthyroid patients, lower serum-free thyroxine levels are associated with higher total cholesterol levels, LDL, and TAG levels. In addition to abnormal serum lipids, the risk of nonalcoholic fatty liver disease (NAFLD) increases with lower free thyroxine levels. As free thyroxine rises, the risk of NAFLD is reduced. This has led to numerous animal studies and clinical trials investigating TH analogs and TH receptor agonists as potential therapies for NAFLD and hyperlipidemia. Thus, TH plays an important role in maintaining hepatic homeostasis, and this continues to be an important area of study. A review of TH action and TH actions on the liver will be presented here.
TH resistance
 Papers TSH NCoR
Papers TSH NCoR
The Nuclear Receptor Corepressor (NCoR) Controls Thyroid Hormone Sensitivity and the Set Point of the Hypothalamic-Pituitary-Thyroid Axis. 2008
The role of nuclear receptor corepressor (NCoR) in thyroid hormone (TH) action has been difficult to discern because global deletion of NCoR is embryonic lethal. To circumvent this, we developed mice that globally express a modified NCoR protein (NCoRΔID) that cannot be recruited to the thyroid hormone receptor (TR). These mice present with low serum T(4) and T(3) concentrations accompanied by normal TSH levels, suggesting central hypothyroidism. However, they grow normally and have increased energy expenditure and normal or elevated TR-target gene expression across multiple tissues, which is not consistent with hypothyroidism. Although these findings imply an increased peripheral sensitivity to TH, the hypothalamic-pituitary-thyroid axis is not more sensitive to acute changes in TH concentrations but appears to be reset to recognize the reduced TH levels as normal. Furthermore, the thyroid gland itself, although normal in size, has reduced levels of nonthyroglobulin-bound T(4) and T(3) and demonstrates decreased responsiveness to TSH. Thus, the TR-NCoR interaction controls systemic TH sensitivity as well as the set point at all levels of the hypothalamic-pituitary-thyroid axis. These findings suggest that NCoR levels could alter cell-specific TH action that would not be reflected by th
Hypothyroidism