Vanessa Franzin
Giulia Durando
STEVIA REBAUDIANA

PLANT DESCRIPTION
Stevia rebaudiana is a perennial small herbaceous shrub plant, whose leaves have significant sweetening proprieties. It is a member of Asteraceae family (the same of lettuce, radicchio, artichoke and
chamomile), and it grows on the mountains between Brazil and Paraguay.
It was described first by the Swiss birth, but Paraguayan adopted, Moises Santiago Bertoni, as Eupatorium rebaudianum; after, the English researcher William Botting Hemsley classified it in Stevia genus, which was discovered by the Spanish botanist and physicist Pedro Jaime Esteve (from whom the name Stevia). The species name, Rebaudiana, was attributed in honour of the chemist Rebaudi, who first studied plant sweetening substance. It is commonly named sweetleaf or sugarleaf.
This plant has not many-side metabolic needs: it grows on poor sandy fields, where there's a surface water-bearing stratum, such as swamps edge and grasslands. In temperature matter it seems to have a quite wide range: its optimum is 23 ーC, but it tolerates also next to 0 ーC values, as well soil acidity.
Stevia has a story longer than 1500 years, linked to Guaranì people (they call it ka'a he'), who mainly live in Paraguay and Brazil. These people use leaves, thirty times as sweet as sugar, in food, tea and infusions, including mate, or simply chewing them; furthermore it has commonly been using for centuries until today from South America natives because of its curative skills.
MAIN CONSTITUENTS AND THEIR USE
Stevia is a genus of about 240 species, but rebaudiana is the only sweetening one. Leaves contain a diterpenoid glycosides set: stevioside and rebaudioside mainly, isolated in 1931 by two French chemist, Briedel and Laivielle. These compounds have a distinctive structure of 3 glucose molecules, and physical and sensory properties deeply characterized: a sweetening skill 300/440 times as sweet as sucrose, they are heat stable and pH stable and non fermentable, this means that they preserve in bakery products and hot drinks, unlike other synthetic sweeteners such as
aspartame, which degrades. Contrary to the sugar these active principles have no nutritional power.
The exact structure of the aglycone and the glycoside were published in 1955.
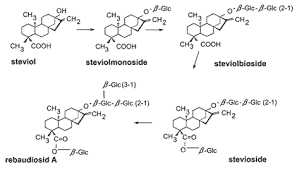
While steviol is an aglucon, rebaudioside A contain 4 glucose molecules versus 3 in stevioside. Rebaudioside A has the least bitterness of all the steviol glycosides in the stevia plant. To produce rebaudioside A commercially, stevia plants are dried and subjected to a water extraction process. This crude extract contains about 50% rebaudioside A; its various glycoside molecules are separated via crystallization techniques, typically using ethanol or methanol as solvent. This allows the manufacturer to isolate pure rebaudioside A.
Stevioside, Rebaudoside C and F, Dulcoside A and Steviolbioside have also been isolated from S. Rebaudiana and are widely used as sweeteners. The sweet diterpenoid glycoside, rebaudioside F has been isolated from leaves and its structure was established by chemical and spectral studies.
The National Research Council of Canada has patented a process for extracting sweet compounds from stevia by column extraction at temperatures from 0–25 °C, followed by purification by nanofiltration. A microfiltration pretreatment step is used to clarify the extract. Purification is by ultrafiltration followed by nanofiltration.
In the early 1970s, Japan began cultivating stevia as an alternative to artificial sweeteners such as cyclamate and saccharin, which were suspected carcinogens. The plant's leaves, the aqueous extract of the leaves, and purified steviosides are used as sweeteners. Steviol glycosides were first commercialized as a sweetener in 1971 by the Japanese firm Morita Kagaku Kogyo Co., Ltd., a leading stevia extract producer in Japan, so they have been using stevia in food products, soft drinks (including Coca Cola), and for table use. Japan currently consumes more stevia than any other country, with stevia accounting for 40% of the sweetener market.
In the last five years, both Coca Cola and PepsiCo have introduced products that contain their new sweeteners. In fact, they have recorded two sweetener brands: Truvia and PureVia.

CLASSIFICATION
- Division: Magnoliophyta
- Class: Magnoliopsida
- SubClass: Euasterid II
- Order: Asterales
- Family: Asteraceae Giseke
- SubFamily: Asteroideae
- Genus: Stevia
- Species: S. Rebaudiana
MOLECULAR MECHANISM
Stevioside absorption, distribution, and metabolism have been evaluated in experimental animals. Absorption of stevioside by everted sacs of rat intestine and Caco-2 cell monolayers was very poor, whereas its aglycone, steviol, was rapidly absorbed. Since stevioside can be degraded to steviol by intestinal microflora from various animal species, including human, it has been suggested that following stevioside consumption only steviol is absorbed. Furthermore, it appears that stevioside shows nearly complete metabolic conversion to steviol in vivo. Thus, the “acceptable daily intake” of 7.9 mg/kg b.wt./day stevioside suggested by
Xili et al., 1992 would yield a maximum plasma concentration of approximately 0.2 mM steviol, assuming that oral stevioside would be fully converted to steviol and fully absorbed by the gut in vivo.
A recent in vivo study in rats gave plasma values in the same range.
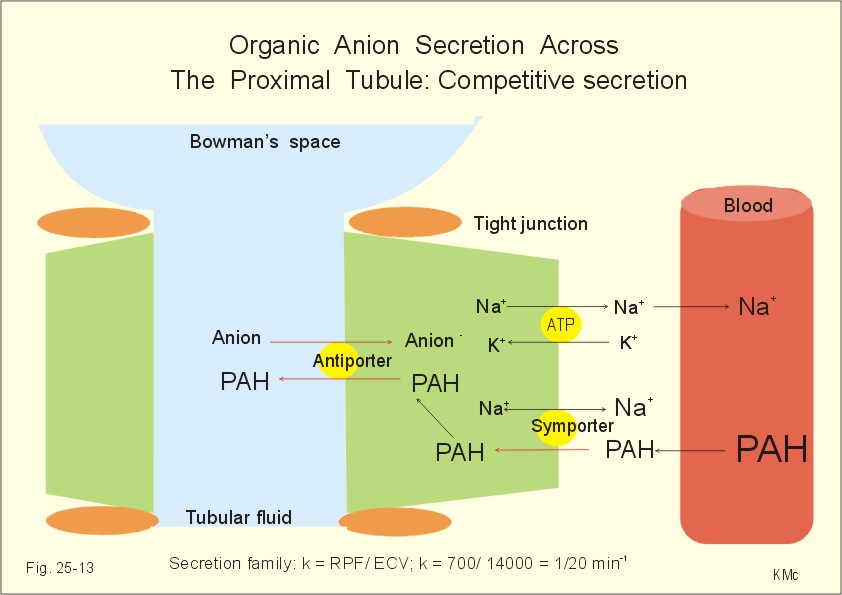
In addition, stevioside and its metabolites are very rapidly eliminated from the body. For example, following intravenous injection of stevioside into the rat, plasma radioactivity decreased rapidly to only 6% of injected dose in the first 60 min. The excreted label was equally found in urine and feces. Moreover, in the rat, stevioside renal clearance was higher than inulin clearance, suggesting active tubular secretion of stevioside (or its aglycone) by renal tubular epithelium. The aim of a lot of studies was to determine which of the cloned basolateral organic anion transporters were involved in the renal transport of stevioside and steviol. This question was addressed in Xenopus laevis oocytes expressing human organic anion transporter 1 (hOAT1), 3 (hOAT3), and winter flounder
OAT (fOat1). The parent compound, stevioside, had no inhibitory effect on either
PAH (hOAT1) or ES (estrone sulfate; hOAT3) uptake.
 | Inhibition of hOAT1-mediated [3H]PAH uptake (A) and hOAT3-mediated [3H]ES uptake (B). Oocytes expressing hOAT1 (A) and hOAT3 (B) were incubated with OR-2 buffer containing either 10 μM[3H]PAH or 100 nM [3H]ES in the absence (control) or presence of 100 μM test compounds for 60 min. The results are expressed as a mean percentage of control from three separate experiments ± S.E. Mean control PAH and ES uptake were 22.1 ± 2.1 and 0.7 ± 0.1 pmol/oocyte/h, respectively. **, p < 0.01; ***, p < 0.001. |
 | Concentration dependence of stevioside and steviol inhibition of hOAT1- and hOAT3-mediated uptake. Oocytes expressing hOAT1 (A) and hOAT3 (B) were incubated for 60 min with OR-2 buffer containing either 10 μM [3H]PAH (A) or 100 nM [3H]ES (B) in the absence (control) or presence of various concentrations of stevioside and steviol. Unlabeled PAH (1 mM) and probenecid (1 mM) were used to illustrate complete block of mediated PAH and ES uptake. Water-injected oocytes showed minimal PAH and ES uptake. The values are expressed as a mean percentage of control from three separate experiments ± S.E. Mean control PAH and ES uptake were 47.5 ± 8.3 and 0.6 ± 0.2 pmol/oocyte/h, respectively. *, p < 0.05; ***, p < 0.001. |
Stevioside itself did not inhibit either hOAT1 or hOAT3. Likewise, our electrophysiological analysis showed that stevioside did not produce membrane currents in oocytes expressing fOat1, suggesting that stevioside had no interaction with either hOAT1 and hOAT3 and thus, that these
OATs are unlikely to mediate basolateral uptake of stevioside. However, this finding is in contrast with previous in vivo studies that found urinary stevioside clearance to be greater than that of inulin. First, it is possible that the stevioside used in the earlier studies contained other stevioside derivatives or that some stevioside metabolism occurred in vivo after its injection. Alternatively, another transport process may be responsible for these in vivo findings. This basolateral transporter appears to handle organic anions, as well as digoxin and ouabain, large neutral cardiac glycosides. Hence,
OATP4C1 may play a possible role for transport stevioside.

In contrast, steviol, gave very different results. Steviol very effectively inhibited hOAT1- and hOAT3-mediated transport in a dose-dependent manner. The inhibition of
OAT1 produced by steviol was as great as that produced by equal concentrations of unlabeled
PAH and furosemide, suggesting that the affinity of steviol approximates that of these compounds for hOAT1. Indeed, it was nearly as effective against hOAT1 as probenecid. Against hOAT3, it was a more effective inhibitor than
PAH, but it was much less effective than estrone sulfate, bumetanide, or probenecid , all of which are known to have a high affinity for hOAT3 However, these cis-inhibition experiments only indicate that steviol has a high affinity for basolateral
OAT binding and leave open the question of whether or not steviol is itself translocated by the cloned
OATs. However, at higher concentrations steviol trans-inhibited [3H]
PAH efflux, similar to the effect of probenecid which binds very tightly to the carrier. The reason for differences in the effects of low (1 μM) and high (100 μM) steviol concentrations on
PAH efflux is not clear, but may indicate that steviol translocation has a low turnover rate. Thus, at high concentrations it may mask the binding site and, like probenecid, produce trans-inhibition. Indeed, that rate of steviol translocation is clearly lower than that of
PAH. This finding is similar to other, who showed that the tightly bound substrate, tetrapentylammonium, completely blocked
TEA efflux from isolated rabbit proximal tubules. These investigators proposed that tetrapentylammonium binds to the organic cation transporter and markedly slows carrier turnover rate.
The recent studies of Jutabha et al. (2000) suggest that inhibition of organic anion transport also occurs at the tubular level. They found that 0.7 mM stevioside modestly reduced
PAH transport, whereas only 10 μM steviol was needed to significantly inhibit transepithelial
PAH transport by the rabbit renal proximal tubule. However, those studies did not assess the interactions of stevioside and steviol with specific basolateral
OATs.
J Pharmacol Exp Ther. 2005 May;313(2):621-8. Epub 2005 Jan 11.
ANTIDIABETIC ACTIVITY
This antidiabetic activity was studied in alloxan-induced
diabetic rats using three types of extracts:
- Aqueous extract - 50 g of powdered leaves were kept in a beaker to which 250 ml of distilled water was added. The mixture was shaken properly and kept at room temperature for 24 h. It was stirred 2-3 times a day. After 24 h, mixture was filtered through ordinary filter paper and the filtrate was evaporated using rotary vacuum evaporator at 40-45°C. The extractability percentage was determined as per the method suggested by Rosenthaler.
- Ether extract - 50 g dried powdered leaves of S. rebaudiana was taken into thimble and 750 ml of petroleum ether was added into the soxhlet and boiled on the water bath. After 10-15 cycles, it was decanted into the beaker and was evaporated using rotary vacuum evaporator at 40-45°C.
- Methanol extract (ME) - 50 g dried powdered leaves of S. rebaudiana was taken into thimble and 750 ml of methanol was taken into the flask of soxhlet apparatus and cycled 10-15 times. After that it was decanted into the beaker and was left open, so that the methanol evaporated using rotary vacuum evaporator at 40-45°C.

The blood glucose levels were 220.16 ± 8.63, 220.00 ± 11.20 mg/dl in AE-, 209.66 ± 4.15, 220.83 ±09.24 mg/dl in EE-, 218.66 ±4.93, 232.00 ± 11.81 mg/dl in ME-treated rats on day 0. In the glibenclamide-treated group, the blood glucose was 211.00 ± 5.10 mg/dl on day 0, whereas in glibenclamide + AE treated rats the blood glucose level on day 0 was found to be 208.16 ± 9.23 mg/dl. It may be noted in the above table that a significant decrease in the mean blood glucose levels was found on day 28 in AE-, EE-, and ME-treated rats, both after 50 and 100 mg/kg daily dose administration. The results obtained in this study for extracts of S. rebaudiana showed decrease in the mean blood glucose levels.
The study also showed that the rats which had been given the extracts of AE and EE at higher dose (100 mg/kg) exhibited greater decrease in mean blood glucose level as compared to those given at a rate of 50 mg/kg b.w. on day 28. Therefore, it is obvious from the results obtained in this study that antihyperglycemic activity of AE and EE were dose-dependent. The data showed that there was significant decrease in the mean blood glucose level (100.50 ± 0.22) in the group VII where AE was given with glibenclamide. However, it did not differ with the blood glucose level on day 28 (101.83±0.30) as compared to that group which received only glibenclamide. Therefore, it is obvious that glibenclamide and AE both are working differently in rats.
The stevia leaves powder has also been reported to reduce the blood glucose concentration of diabetic rats. Some experiment was undertaken to clarify the participation of steviol on the Na+ - glucose renal tubular transport system. After a control period steviol was infused in three doses: 0.5, 1.0, 3.0 mg/kg/h.
During all the experiments no significant changes in inulin clearance and p-aminohipuric acid clearance were observed. Administration of steviol resulted in a statistically significant increase in the fractional sodium excretion, fractional potassium excretion, urinary flow as percent of glomerular filtration rate and glucose clearance, when compared to controls, but these effects were absent with the dose of 0.5 mg/kg/h. the data suggested that steviol causes diuresis, natriuresis, kaliuresis and a fall in renal tubular reabsorption of glucose.
It was concluded that the extracts of S. rebaudiana could decrease the blood glucose level in diabetic rats in time-dependent manner. The antidiabetic effect might be due to steviosides counteracting the glucotoxicity in β-cells or also by suppressing the glucagon secretion by α-cell of pancreas.
Another study demonstrated that stevioside stimulates the insulin secretion both in vitro and in vivo and the influence of rebaudioside A on the insulin release from mouse islets using static incubations, as well as perifusion experiments. The study is the first to demonstrate that rebaudioside A causes a dose- and glucose-dependent stimulation of insulin secretion from isolated mice islets. Rebaudioside A elicits a typical monophasic insulin response. Interestingly, the stimulatory effect of rebaudioside A disappears in the presence of normal or low glucose. In the absence of extracellular calcium, the insulinotropic effect of rebaudioside A vanishes.
 | Effect of rebaudioside A (10−10 mmol/L, ■) on insulin secretion from isolated mouse islets in the presence of glucose concentrations of 3.3, 6.6, 11.1, and 16.7 mmol/L, respectively. #P < .05 v control (in the absence of rebaudioside A, □). Each bar represents the mean ± SEM from 16 incubations of a single islet. |
 | Insulin secretion from perifused isolated mouse islets in the absence (control, ○) or presence of rebaudioside A (10−10 mol/L, •) at low (3.3 mmol/L) and high (16.7 mmol/L) glucose. Each curve is the mean ± SEM from 6 perifusion experiments with 25 islets in each. |
 | Effect of rebaudioside (10−10 mol/L) on insulin release in Ca2+-free medium. Isolated mouse islets were incubated for 60 minutes in the absence or presence (control) of extracellular calcium at glucose concentrations of 3.3 (■), 16.7 (□), and 16.7 mmol/L plus rebaudioside A (10−10mol/L, ▩). Each bar represents the mean ± SEM from 16 incubations of single islets. #P < .05 v control. |
Rebaudioside A seems to be more potent than stevioside and steviol, eliciting clear-cut insulin stimulation even at a concentration as low as 10−14 mol/L. The reason for the higher potency of rebaudioside A is not known. The difference in number and positions of the glucose molecules in these glycoside molecules may play a role in this respect. To understand the mechanisms of action of rebaudioside A on the β cells, the researchers incubated pancreatic islets with diazoxide, a compound that inhibits the insulin release by increasing K+ conductance of the β-cell membrane through opening of the adenosine triphosphate (ATP)-sensitive K+ channels and Rebaudioside A was unable to reverse the inhibitory effect of 200 μmol/L diazoxide on glucose-induced insulin release. This suggests that rebaudioside A may not affect ATP K+ channels in the β cells like we previously demonstrated for stevioside. Further studies are, however, needed to clarify whether or not rebaudioside A does affect the ATP K+ channels directly.
In the present study, rebaudioside A (10−10 mol/L) did not stimulate insulin release at 16.7 mmol/L glucose in the absence of extracellular Ca2+. This suggests that the insulinotropic effect of rebaudioside A is critically dependent on Ca2+ influx from the extracellular space. At first glance this may occur puzzling considering our previous findings indicating that the insulinotropic effect of both stevioside and steviol is preserved in the absence of extracellular Ca2+. However, it is noteworthy that the diterpene concentration needed to stimulate the insulin secretion in a Ca2+ medium was extremely high (10−3 to 10−2 mol/L). Stevioside, as well as steviol, in a concentration causing maximal effect (10−6 mol/L) were unable to enhance the insulin release in the absence of extracellular Ca2+. The augmentation of the insulin release in Ca2+ medium in previous studies may consequently be due to unspecific effects at extremely high levels of the glucosides. It seems unlikely that it is ascribed to an irreversible toxic effect on β cells, because the islets subsequently responded with a normal insulin response to carbacholine in the presence of normal extracellular calcium during a perifusion study.
In conclusion, rebaudioside A potently stimulates the insulin secretion from isolated mouse islets in a dose- and glucose-dependent manner. Interestingly, rebaudioside A does not cause a stimulation of insulin release at near-normal glucose levels, which is likely to reduce or eliminate the risk of hypoglycemia. Whether rebaudioside A may serve in the treatment or prevention of type 2 diabetes remains to be elucidated.
The results of other researches suggest that effects on blood glucose levels may be different depending on the status of the subjects and that there are differences in diabetic and non-diabetic animals and humans.
This study was designed to evaluate the effects of steviol glycosides on blood glucose and on blood pressure (BP) in 3 groups of individuals. This was a randomized, double-blind, placebo-controlled, long-term study in three groups of patients: Group 1: subjects with Type 1 diabetes; Group 2: subjects with Type 2 diabetes; and Group 3: subjects without diabetes and with normal/low-normal BP levels. The subjects in each group were randomly allocated to active treatment (the steviol glycoside stevioside: 250 mg t.d.s.) or to placebo treatment and followed-up for 3 months.
Table 1
Characteristics of the steviol glycosides and placebo groups at baseline and post-treatment: Group 1 (Type 1 diabetes)
| Steviol glycosides (n = 8)
| Placebo (n = 8)
|
|---|
| Baseline | Post-treatment | Baseline | Post-treatment |
|---|
| BMI (kg/m2) | 23.2 (3.3) | 23.1 (3.1) | 22.4 (1.0) | 22.4 (1.0) |
| 24-h SBP (mmHg) | 117.1 (6.6)⁎ | 115.9 (8.6) | 108.3 (3.0) | 105.7 (2.8)⁎⁎ |
| 24-h DPB (mmHg) | 72.6 (6.9) | 68.9 (7.2) | 70.7 (4.4) | 69.7 (3.3) |
| | | | |
| Laboratory |
| Glucose | 144.9 (95.1) | 155.3 (78.3) | 219.3 (74.1) | 298.3 (58.8)⁎⁎ |
| HbA1c (%) | 7.1 (1.6) | 7.3 (1.1) | 8.2 (1.4) | 8.3 (1.6) |
| Total cholesterol (mg/dl) | 148.9 (25.9) | 159.5 (36.7) | 144.6 (16.4) | 150.6 (10.0) |
| HDL-C (mg/dl) | 46.3 (6.3) | 44.6 (8.0) | 53.0 (7.7) | 48.7 (11.4) |
| LDL-C (mg/dl) | 85.4 (21.8) | 96.3 (27.6) | 81.6 (20.6) | 88.8 (7.4) |
| Triglycerides (mg/dl) | 86.8 (33.6)⁎ | 86.8 (41.5) | 51.3 (11.5) | 67.3 (15.1) |
| Creatinine (mg/dl) | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.2) | 0.9 (0.1) |
p < 0.05 versus placebo baseline.
p < 0.05 post-treatment versus baseline (within treatment group).
Table 2
Characteristics of the steviol glycosides and placebo groups at baseline and post-treatment: Group 2 (Type 2 diabetes)
| Steviol glycosides (n = 15)
| Placebo (n = 15)
|
|---|
| Baseline | Post-treatment | Baseline | Post-treatment |
|---|
| BMI (kg/m2) | 28.7 (3.4) | 29.2 (2.9) | 30.1 (3.3) | 30.2 (3.5) |
| 24-h SBP (mmHg) | 127.3 (15.1) | 124.3 (13.5) | 127.9 (13.7) | 124.9 (13.3) |
| 24-h DPB (mmHg) | 77.3 (9.1) | 74.7 (8.3) | 76.7 (5.6) | 77.4 (9.5) |
| | | | |
| Laboratory |
| Glucose (mg/dl) | 151.2 (54) | 133.8 (34.5) | 131.3 (46.7) | 118.9 (34.0) |
| Insulin (μUI/ml) | 13.3 (15.3) | 11.6 (11.1) | 14.7 (10.2) | 15.3 (9.6) |
| HbA1c (%) | 6.8 (1.2) | 6.6 (1.1) | 6.8 (1.6) | 6.8 (1.0) |
| Total cholesterol (mg/dl) | 186.9 (23.3) | 175.1 (21.6) | 172.7 (31.6) | 173.8 (23.0) |
| HDL-C (mg/dl) | 44.6 (8.7) | 42.5 (6.7) | 44.6 (10.9) | 40.8 (6.2) |
| LDL-C (mg/dl) | 111.4 (23.8) | 107.8 (22.2) | 93 (31.8) | 110.2 (18.6) |
| Triglycerides (mg/dl) | 143.2 (51.8) | 153.7 (71.3) | 133.8 (53.3) | 128.1 (39.2) |
| Creatinine (mg/dl) | 0.9 (0.1) | 0.9 (0.3) | 0.9 (0.2) | 0.8 (0.2) |
Table 3
. Characteristics of the steviol glycosides and placebo groups at baseline and post-treatment: Group 3 (non-diabetics with normal/low-normal BP)
| Steviol glycosides (n = 13)
| Placebo (n = 17)
|
|---|
| Baseline | Post-treatment | Baseline | Post-treatment |
|---|
| BMI (kg/m2) | 22.9 (2.7) | 23.2 (2.7) | 24.4 (3.8) | 24.5 (3.5) |
| 24-h SBP (mmHg) | 111.0 (8.9) | 113.3 (10.8) | 111.7 (10.4) | 112.2 (11.9) |
| 24-h DPB (mmHg) | 69.9 (7.2) | 69.8 (7.1) | 68.8 (5.5) | 69.9 (8.1) |
| | | | |
| Laboratory |
| Glucose (mg/dl) | 82.5 (6.6) | 82.9 (7.8) | 82.9 (10.2) | 83.9 (7.0) |
| Insulin (μUI/ml) | 4.2 (1.8) | 4.9 (2.4) | 8.4 (8.1) | 8.7 (8.1) |
| HbA1c (%) | 5.3 (0.4) | 5.6 (0.6) | 5.3 (0.6) | 5.4 (0.7) |
| Total cholesterol (mg/dl) | 164.7 (30.8) | 173.7 (27) | 164.1 (32.5) | 173.5 (29.9) |
| HDL-C (mg/dl) | 51.7 (9.1) | 50.1 (10.5) | 53.2 (8.1) | 52.8 (9.7) |
| LDL-C (mg/dl) | 91.1 (19) | 103.8 (20.3) | 92.6 (27.8) | 98.9 (16.9) |
| Triglycerides (mg/dl) | 96.5 (44.2) | 99.6 (33.2) | 91.8 (62.6) | 108.6 (100.5) |
| Creatinine (mg/dl) | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) | 0.8 (0.1) |
In vivo studies in diabetic rats have demonstrated a decrease in blood glucose after stevioside administration (25 mg/kg/day stevioside for 6 weeks). However, these effects of oral stevioside or steviol glycosides are not seen in non-diabetic animals. A long-term chronic toxicity study found no change in blood glucose in rats fed a diet containing 1.2% stevioside for 24 months, and another one found no change in blood glucose in an acute oral study.
Similarly, studies in diabetic humans have demonstrated a decrease in blood glucose after acute stevioside administration (1 g with a meal) found an increase in fasting blood glucose in diabetic patients receiving placebo but not those receiving 500 mg stevioside 3 times daily for 3 months. Two studies with Stevia extract in normal humans have demonstrated an apparent dose-related decrease in blood glucose but others effect of much larger doses of stevioside (250 mg 3 times daily for 1 year) on fasting blood glucose in hypertensive, non-diabetic subjects. The fact they didn’t find a reduction in blood glucose at relatively high doses of stevioside suggests that perhaps the effect seen at lower doses was due to some other mechanism. The present results support these conclusions, as no statistically significant change was observed in the post-treatment mean glucose and HbA1c levels in the steviol glycoside group.
The same studies have examined the effect of oral steviol glycosides on normal and hypertensive humans and animals.
R. S. Kujur et al. July aug. 2010
ANTI-HYPERTENSIVE EFFECT
In normal and hypertensive rats and normal dogs, a small but significant reduction in blood pressure (either mean arterial pressure or systolic and diastolic pressure) has been observed. A blood pressure lowering effect has been previously shown in chronic studies in humans using 250 mg 3 times daily (the same dose used in our study) when given to patients with high blood pressure for 1 year or 500 mg 3 times daily for 2 years . However, in a more recent study in Type 2 diabetic patients, no effect on blood pressure was found when stevioside was given at much higher doses (500 mg 3 times daily for 3 months); similarly in non-hypertensive subjects, using 250 mg 3 times daily for 3 days wasn’t find any effect on blood pressure. Other studies did not find any effect on blood pressure at doses up to 15 mg/kg bw/day in mildly hypertensive humans
Other studies examined the antihypertensive effect of crude stevioside on previously untreated mild hypertensive patients, who were submitted to a placebo phase for 4 weeks.The volunteers selected in this phase were randomly assigned to receive either capsules containing placebo during 24 weeks or crude stevioside 3.75 mg/kg/day (7 weeks), 7.5 mg/kg/day (11 weeks) and 15.0 mg/kg/day (6 weeks). All capsules were prescribed twice a daily (b.i.d.), i.e. before lunch and before dinner. After the placebo phase and after each dose of crude stevioside, body mass index, electrocardiogram and laboratory tests were performed. During the investigation blood pressure (BP) was measured biweekly and the remaining data were collected at the end of each stevioside dose step. Systolic and diastolic BP decreased (p < 0.05) during the treatment with crude. stevioside, but a similar effect was observed in the placebo group. Therefore, crude stevioside up to 15.0 mg/kg/day did not show an antihypertensive effect. Moreover, the results suggest that oral crude stevioside is safe and supports the well-established tolerability during long term use as a sweetener in Brazil.
Table 4
Systolic (SBP) and diastolic blood pressure (DBP) before (phase 0) and after treatment with crude stevioside 3.75 mg/kg/day (phase 1), 7.5 mg/ kg/day (phase 2) and 15.0 mg/kg/day (phase 3)
| Phase | 0 | 1 | 2 | 3 |
| Stevioside SBP | 140 ± 13 | 134 ± 14 | 126 ± 8a | 123 ± 12 |
| Stevioside DBP | 94 ± 8 | 85 ± 5a | 84 ± 5a | 84 ± 8a |
| Placebo SBP | 133 ± 12 | 128 ± 5 | 132 ± 6 | 124 ± 6 |
| Placebo DBP | 94 ± 8 | 86 ± 3a | 83 ± 5a | 82 ± 4a |
Values (mmHg) are mean ± SD (n = 6). p > 0.05 for all comparisons (Stevioside group vs Placebo group). a p < 0.05 compared with phase 0.
During the treatment DBP (p < 0.05) decreased not only in the patients who received crude stevioside but also in the placebo group (Table 1). It could be inferred that this effect is a response to the presence of a physician during the clinical visits, i.e. an opposite effect of white coat hypertension. However, several investigations showed that stevioside decreased BP in rats. But it must be emphasized that in those reports parenteral administration of stevioside was employed. In addition, a discrete reduction of BP was also obtained by nasogastric administration of stevioside in dogs, but the dose was very high, i.e. 200 mg/kg. On the other hand, the antihypertensive effect of oral crude stevioside was obtained in two placebo doubleblind clinical trials. Since stevioside is degraded by intestinal microflora of rats, pigs and humans to the diterpenoid aglycone steviol, the antihypertensive effect of orally administered stevioside could be mediated by steviol. Nonetheless, the presence of steviol in the blood after oral ingestion of stevioside was not confirmed. The absence of a hypotensive effect could be a consequence of our lower basal values of BP (pre hypertension and stage I); the small number of patients; higher BMI; race and/or frequency of daily ingestion of the capsules (twice a day vs thrice a day in Chan’s study). Another possibility could be the composition of crude stevioside. The blood levels of testosterone, estradiol, free and total PSA were not modified by crude stevioside treatment. In contrast, previous reports demonstrated that leaves of Stevia rebaudiana Bertoni, which contain not only steviol glycosides but also thousands of compounds affected the reproductive system. Therefore, the conclusions obtained from the leaves of Stevia rebaudiana Bertoni, cannot be expanded to crude stevioside. In contrast to this data, stevioside´s potential for sodium excretion in rats has been described.
Nevertheless, in these reports the stevioside was administered intravenously. Data also shows decreased (p < 0.05) blood levels of total cholesterol, LDL-C, VLDL-C, triacylglycerol, glucose and insulin (phase 3 versus phase 0). In agreement with these results HOMA IR (Table 2) and LDLC/HDL-C ratio (not shown) were decreased (p < 0.05) (phase 3 versus phase 0). Taken together, these findings suggested an improvement of insulin action during the treatment not only with crude stevioside but also with placebo. Thus, in spite of the fact that all patients were instructed to maintain their lifestyle during the trial, the results were compatible with modifications in the lifestyle during the study.
Leticia A.F. Ferri et al. June 2006
TOXICITY AND POLITICAL CONTROVERSY
In 1991, after receiving an anonymous industry complaint, the United States Food and Drug Administration labeled stevia as an "unsafe food additive" and restricted its import. The FDA's stated reason was "toxicological information on stevia is inadequate to demonstrate its safety." This ruling was controversial, as stevia proponents pointed out that this designation violated the FDA's own guidelines under which natural substances used prior to 1958, with no reported adverse effects, should be generally recognized as safe (GRAS) as long as the substance was being used in the same way and format as prior to 1958.
Stevia, the plant, is ineligible as a natural substance for patent protection. A process for extracting its "active ingredient" could, all other legal requirements being met, be patented. As a consequence, since the import ban in 1991, marketers and consumers of stevia have shared a belief that the FDA acted in response to industry pressure.
Stevia remained banned until after the 1994 Dietary Supplement Health and Education Act forced the FDA in 1995 to revise its stance to permit stevia to be used as a dietary supplement, although not as a food additive — a position that stevia proponents regard as contradictory because it simultaneously labels stevia as safe and unsafe, depending on how it is sold.
Unresolved questions remain about whether metabolic processes can produce a mutagen from stevia in animals. Early studies prompted the European Commission in 1999 to ban stevia's use in food in the European Union pending further research. More recent data compiled in the safety evaluation released by the World Health Organization in 2006 suggest that these policies may be obsolete. Since 2008, the Russian Federation has allowed stevioside as a food additive "in the minimal dosage required".
Reb A was investigated (Burdock Group Aug 2009) for its potential to induce genotoxicity in three in vitro and two in vivo assays (conducted according to OECD guidelines). Reb A was non-mutagenic in an Ames test using Salmonella typhimurium and Escherichia coli, in a chromosomal aberration test using Chinese Hamster V79 cells and in a mouse lymphoma assay using L5178Y+/- cells, all studies were conducted at concentrations up to 5000 microg/ml, with and without metabolic activation. Also, Reb A was non-genotoxic in a bone marrow micronucleus test in mice at doses up 750 mg/kg bw and in an unscheduled DNA synthesis test in rats at 2000 mg/kg bw. These studies provide additional evidence that Reb A is not genotoxic at the doses tested and further support the generally recognized as safe determination of Reb A.
In December 2008, the FDA gave a no objection approval for GRAS status to Truvia (developed by Cargill and The Coca-Cola Company) and PureVia (developed by PepsiCo and the Whole Earth Sweetener Company, a subsidiary of Merisant), both of which use rebaudioside A derived from the Stevia plant.
The European Food Safety Authority evaluated the safety of steviol glycosides, extracted from the leaves of the Stevia rebaudiana Bertoni plant, as sweetener and expressed its opinion on 10 March 2010. The Authority established an Acceptable Daily Intake (ADI) for steviol glycosides, expressed as steviol equivalents, of 4 mg/kg bodyweight/day. On 11 November 2011, the European Commission allowed the usage of steviol glycosides as food additive, establishing maximum content levels for different types of foods and beverages.
