Thromboembolic Disease in Pancreatic Cancer
Pancreatic cancer is still a major clinical challenge.
Recent efforts to improve survival in locally advanced and metastatic disease have focused on combining cytotoxic drugs with targeted therapies. It is also one of the solid tumors with the highest incidence rate of venous thromboembolism (VTE), which represents one of the major complications.
Despite the high incidence of thromboembolic complications, there is little data regarding the incidence and pathogenesis of VTE in pancreatic cancer patients.
Clinical data suggest that, among patients with unresectable pancreatic cancer, the occurrence of VTE may be associated with reduced overall survival. Furthermore emerging clinical data strongly suggest that anticoagulant treatments may improve cancer patient survival by decreasing thromboembolic complications as well as by anticancer effects.
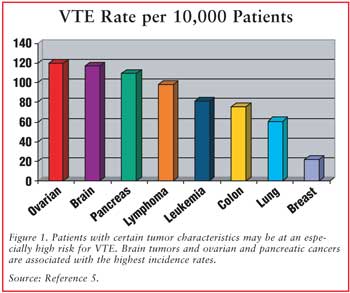
For more information: Venous thromboembolism and pancreatic cancer: incidence, pathogenesis and clinical implications.
Thromboembolic disease in pancreatic cancer presents a life-threatening (but common) complication in pancreas cancer and is regarded as paraneoplastic manifestation of the disease. It is causally associated with the generation of an intrinsic hypercoagulable state. Pancreatic cancer cells activate platelets and express several procoagulant factors, including tissue factor (TF, the physiological initiator of blood coagulation, over-expressed in pancreatic cancer) and thrombin.
It seems that high circulating levels of tissue factor are associated with this pro-coagulant and pro-angiogenic state in the cancer. TF associates with microparticles (MP) , derived from cellular death; MP-TF activity is associated with thrombosis and worsened survival in patients with pancreatico-biliary cancer.
Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers.
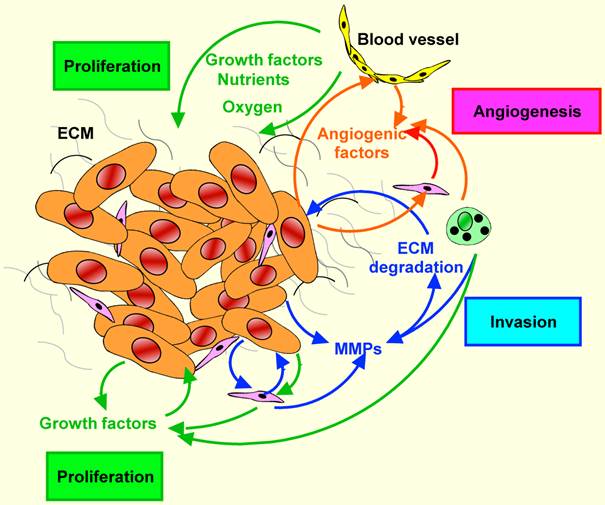
The activation of coagulation is not simply an epiphenomenon, but might also be related to enhanced tumour growth and angiogenesis.
Clinical manifestations of thromboembolic disease in pancreatic cancer include deep venous thrombosis, pulmonary embolism, disseminated intravascular coagulation, portal vein thrombosis, and arterial thromboembolism.
Reported incidences of disease range from 17% to 57%.
Treatment options include warfarin and low-molecular-weight heparins (LMWH). Studies over the past decade suggest that long-term use of these heparins in both primary and secondary prevention of venous thromboembolic disease improves outcomes in comparison with warfarin.
LMWH are preferrable to unfractioned heparins because are less susceptible to inactivation by heparin-binding proteins.
For more information, see: High affinity binding of heparin by necrotic tumour cells neutralises anticoagulant activity--implications for cancer related thromboembolism and heparin therapy.
Anyway, both warfarin and heparin are used in prevention of VTE and they seem to increase the mean survival.
Role of anticoagulation in the management of pancreatic cancer
More about thrombosis and hypercoagulation
Hypercoagulation is common in patients with pancreatic cancer because of tumor location (i.e. Retroperitoneal), decreased activity in bedridden patients, frequent hospitalisation and radiation injury to the vessels.

(Source)
Moreover, in pancreatic cancer, tissue factor, thrombin and fibrinogen level are increased, while the inhibitors are decreased. In this situation platelets aggregation increases deeply and results from the increased expression of fibrinogen and thrombospondin-1, as well as the increased productions of mucins by the tumor (mucins interact with the platelets). The platelet-rich aggregates then are more likely to cause microangiopathic disease.
Also inflammation is involved in the cancer-related VTE. TGF is upregulated in the carcinoma cell lines and it induces Plasminogen Activator Inhibitor-1 (PAI1), which has a pro-coagulatory effect. TNF is antoher cytokine upregulated in various types of cancers; it also induces the tissue factor and downregolates the thrombomodulin (inhibitor of coagulation) in endothelial cells. At the moment there isn't a significative evidence that the TNF expression in pancreatic carcinoma cells induces these effects on coagulation, but inflammatory cells recruited in the tissue contribute to the angiogenesis . This could also explain the fact that VEGF is expressed by mast cells and macrophages .
Also genetical factors can contribute, like the activation of K-Ras, often associated with the inactivation of p53 (70%).
These two proteins have a cumulative effect in the induction of TF expression.

(Source )
Sometimes thrombotic event could be the first presentation of cancers (reported in 3.3-5.0% of patients).
Recent studies evidenced that malignant growth has also been linked to activity of heparin-like glycosoaminoglycans, to neoangiogenesis, to protease activity, to immune function and gene expression in addition with activation of coagulation and fibrinolysis. Emboli from the tumor can cause stroke and pulmonary embolism.
Role of Anticoagulation in the Management of Pancreatic Cancer
In fact, thrombosis is a common complication of malignant diseases and pulmonary embolism is the second commonest cause of death in cancer patients. In pancreas adenocarcinoma, the expression of the cell surface procoagulant tissue factor (TF) correlates with histological grade; these cells also possess a high prothrombinase activity, a prerequisite to efficient cell surface thrombin generation.
Thrombin is generated by tumour cells in large quantities, therefore explaining the peritumoral fibrin deposition and providing a possible mechanism for the hypercoagulable state seen in cancer patients. Moreover, in tumor cells thrombin promotes the sinthesis and release of urokinase plasminogen activator.
It is also an important mitogen to normal cells and also to cancer cells, potentiating the proliferative response to insulin , EGF , and transferrin .
Other activated serine proteases generated during the activation of coagulation have roles outside normal haemostasis and are mitogenic. Of particular interest is factor Xa , the factor occupying the pivotal position in the normal coagulation pathway. Recently a specific receptor (effector cell protease receptor-l, EPR-1) for this factor has been identified and cloned and shown to be of functional significance.
Neither factor Xa receptor nor thrombin receptor have previously been described in any malignant cell.

(Source)
Expression of cell surface tissue factor results in the factor-VIIa-dependent activation of factor X. This complex of activated factors X, V, calcium ions and cell surface phospholipid is responsible for the conversion of prothrombin to thrombin. The prothrombinase complex represents an efficient amplification mechanism for the activation of thrombin and, in the case of the cell surface, a clear method for localisation of the generation of this serine protease.
Factor Xa receptor may play a role in both localising and enhancing the prothrombinase actions of factor Xa.
The thrombin receptor , widely distributed, is of particular interest on tumour cells. Thrombin plays an important role in tumours, enhances both metastatic potential in vivo (studies with animal models) and tumour cell adhesion in vitro, but the mechanisms of its action aren't clear yet.
The colocalisation of these antigens creates an efficient cell surface mechanism for the generation of thrombin in tumour cells.
This cascade (from TF to thrombin receptor) may represent an important autocrine mechanism" in tumours. Interference with this pathway at any level may provide new therapeutic advances, both in modulation of tumour metastatasis and in the prevention of the thrombotic complications of cancer, novel specific antithrombins may have superior effects.
Identification of a thrombin receptor with factor Xa receptor and tissue factor in human pancreatic carcinoma cells
Cytokines (especially IL-6)
A few studies have been made about cytokines in pancreatic carcinoma. The results are the following.
Malignant cells produce a number of cytokines, e.g. IL-1, IL-6, IL-8, IL-13, TNF-α and transforming growth factor β (TGF-β) . Factors such as IL-1β and TNF-α stimulate endothelium to produce tissue factor (TF). Under physiological conditions, the level of TF is strictly regulated in order to maintain the fluidity of blood, but in cancer its expression increases in normal and neoplastic cells in response to proinflammatory factors. Tissue factor activates the coagulation cascade and induces the formation of thrombin, a mitogenic factor, capable of stimulating tumor growth and metastasis. Both thrombin and fibrin, generated as a result of thrombin action, are proangiogenic factors. Angiogenesis-dependent activation of thrombin may occur by pathways dependent on or independent of the blood coagulation cascade. The mechanism depending on the coagulation cascade is triggered by the activation of coagulation proteins and conversion of zymogen prothrombin into proteolytically active thrombin. Induction of angiogenesis by thrombin in the pathway independent of the blood coagulation cascade takes place with thrombin PARs (protease activated receptors) and G proteins.
Thrombin generated in these processes increases the synthesis of many factors, including vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), IL-6, TGF-β, MMP-2 and TF.
Platelets activated by the pro-angiogenic factor VEGF facilitate the penetration of tumor cells through the vessel wall (extravasation) and metastasis.
The role of fibrinogen, fibrin and fibrin(ogen) degradation products (FDPs) in tumor progression.
Circulating levels of VEGF, TNFα, IL-1
and IL-1�β were not elevated significantly in patients with pancreatic carcinoma, but levels of IL-6, IL-8, IL-10, and IL-1 receptor antagonist (IL-1RA) were elevated significantly. Analysis showed that patients who had higher IL-6 levels or IL-10 levels �had significantly worse survival compared with patients who had lower IL-6 or IL-10 levels. IL-8 levels were not associated with survival differences. Patients who had IL-1RA levels
had significantly worse survival compared with patients who had higher IL-1RA levels. Higher IL-6, IL-10, and IL-8 levels were associated with poor performance status and/or weight loss. In multivariate analysis, only T4 tumors and high IL-6 levels were selected as independent prognostic factors for poor survival.
Circulating levels of several cytokines were high in patients with pancreatic carcinoma, and their association with weight loss and poor performance status suggested that they may be involved in these disease manifestations. Furthermore, serum cytokine levels, in particular IL-6, may be a useful prognostic marker.

Figure: Survival of patients with pancreatic carcinoma stratified according to interleukin-6 (IL-6) levels. Distributions estimated using the Kaplan–Meier method. Tick marks represent the time of last follow-up for patients who remained alive. Patients with high IL-6 levels had worse survival.
Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis.
Conclusions

(Source)
Clinical data also suggest that the occurrence of venous thromboembolism may be associated with poorer prognosis in such patients. Patients who have a thromboembolic event are more likely to have advanced cancer diagnosed within 4-12 months. A synchronous thromboembolism was detected in 19.3% of patients associated with a higher probability of not responding to therapy, but did not predict a shorter survival.
So, it seems reasonable to say that the development of thromboembolic disease around the time of diagnosis might predict poor prognosis in patients with pancreatic carcinoma.
Recent data suggest that anticoagulant treatments may improve cancer patient survival by decreasing thromboembolic complications as well as by anticancer effects.
For further information: Role of anticoagulation in the management of pancreatic cancer