Skin is the larger organ of the body and it covers its entire surface. Because of its location skin is the first tissue that front many environmental agents and for this reason, in the past, it was considered a simple protective layer of the rest of the body.
Now is clear that it has a fundamental function that go beyond the simple role of "shield".
It is known that exposition to solar light induces a series of reactions in the skin, like erythema, hyperalgesia and hyperpigmentation.
Sunlight is composed by a broad spectrum of wavelength, more important of them are the visible light (400-700nm) and UV radiation, distinct in UVA (320-400nm), 95% of total UV, and UVB (290-320nm), remaining 5%.
Physical characteristics of these radiations give them different proprieties: UVB radiation reaches the epidermis and, to a lesser extent, the upper part of the dermis and interacts directly with DNA which results in its damage, while UVA radiation penetrates more deeply into human skin and can also cause DNA damage, but it primarily induces the generation of reactive oxygen species (1) (2).
Studies demonstrates that skin exposition to sunlight leads to a wide range of homeostatic modifications.

Almost 1% or respirated oxygen is physiologically converted into ROS in mitochondria , exposing in this way all aerobic organisms to oxidant stress.
The pathological unregulated ROS production causes directly cell damage, in fact these molecules react unspecifically potentially with all cellular compounds, generating for example peroxydated lipids, denaturated proteins (mainly oxydating disulfide bonds) and bases modification into the DNA chain.
In order to remedy this phenomenon evolution provided to aerobics organism many defensive mechanism to protect themselves from oxidative damage; the most important of them are enzymes SOD , catalase and a variety of peroxydases in addition to numerous antioxydant-activity substances (i.e. GSH , ascorbic acid , vitamin E , bilirubin , uric acid).
Daily skin exposure to solar radiation causes cells to produce reactive oxygen species (ROS), which are a primary factor in skin damage. In fact using clinical models to assess the generation of free radicals from oxidative stress, higher levels of free radical activity were found after visible light exposure (3).
Ultraviolet B (UVB) irradiation has recently been shown to generate a variety of ROS products like the hydroxyl radical (HO) with long term detrimental effects on cells like cancer formation and premature aging of the skin.
Another important indicator of cell exposition to UV radiation is the release of ferric/ferrous iron from ferritin (4) (5).
UVA radiation leads to an immediate measurable increase in free iron in human skin fibroblast and keratinocyte cytoplasm and provide a new insight into UVA-induced skin damage, since iron is a catalyst of biological oxidations. (6) Free cytoplasmatic iron is, in fact, very dangerous for cells: the same facile propriety that permits iron to gain and lose electrons can result in the donation of electrons to oxygen, causing the generation of superoxyde anions and the hydroxyl radical. The one-electron reduction of dioxygen by Fe2+ results in superoxyde formation, which in turn leads to the well-known Haber-Weiss-Fenton (7) (8) sequence generating hydroxyl radical (OH).

Furthermore these phenomena are exacerbated by UVA-mediated destruction of cellular reducing equivalents (9) and denaturation of antioxidant enzymes catalase and superoxide dismutase .
The physiological increasing of iron in female body after menopause, due to the cessation of menstrual fluxes, is responsible for the accelerated aging (1) of women skin in post-menopausal life (5).
Reactive oxygen species (ROS ) are also important second messengers for the induction of several genes in a variety of physiological and pathological conditions; in fact ROS induce the cytoplasmatic enzyme Jun N-terminal kinase 2 (JNK2) (10) (11) activity, that in turn lead to an increase of c-jun and c-fos mRNA levels in cell. Also the nuclear factor kappa-light-chain-enhancer of activated B cells is enhanced by iron-catalyzed lipid secondary messengers formation.
AP-1 (composed by the sterical interaction between c-jun and c-fos) and Nf-Kb are strong inductors of the transcription of a variety of nuclear genes, more important of these are genes that encode for the cytokines IL-1, TNF-α, the matrix-degrading metalloprotease (MMP)-1/interstitial collagenase and MMP-3 (12) in addition to several genes that regulate the cell-cycle .
Moreover it seems that UV rays directly stimulate the activation of the second messenger c-JUN N-terminal kinase-1 (JNK1) (10) (13) .

Another protein whose induction is stimulated by UV irradiation is the enzyme heme-hoxygenase-1 (the HO2-isoform is constitutivelyy expressed in many cell types) that catalyze the formation of biliverdin from hemoglobin and have a important role in protection from oxidative damage and, thus, an antiflogistic activity. (14)
An important consequence of the UV-induced altered redox state of the cell is the generation of less known second messengers: UVA-induced iron increase, combined with ROS formation is responsible of the induction of many oxidized membrane compounds in the form of 4-hydroxynonenal, ceramides and oxidized phospholipids, all of which are potent activators of many signaling molecules (Nf-Kb) which modulate of the expression of many genes involved in flogistic process. (15)
In this way UVR-induced iron release determine an exacerbation of the direct effect of UVR on cell structures.
All these events cause an inflammatory state and can lead to connective tissue degradation, two hallmarks in carcinogenesys and tissue aging (1)
ROLE OF TNF-α AND KERATINOCYTE RESPONSE TO SUNLIGHT

Tumor necrosis factor-α is a proinflammatory cytokine produced inside the skin in response to ultraviolet B radiation (UVB) by mRNA induction in both keratinocytes and dermal fibroblasts.
Paracrine effects of this molecule is enhanced by IL1 , another important cytokine that acts sinergically producing an increased effect.
Studies demonstrates that modifications in the promoter region of TNF gene that is the putative binding site for AP-1 and Nf-Kb lead to a lack of synthesis of TNF-α confirming their role in TNF gene induction.
The principal physiologic function of TNF is the stimulation and the recruitment of neutrophils and monocytes to sites of its liberation in order to activate these cells. TNF also stimulates endothelial cells to express adhesion molecules that make the endothelial surface adhesive for leukocytes (selectins and integrins).
In case of very prolonged exposition to sunlight there is a production of a large amounts of this cytokine that lead to its systemic effects like fever (by production of prostaglandins by cytokine-stimulated hypothalamic cells), acute phase protein synthesiss, cachexia, low cardiac output and intravascular thrombosis, that in extreme cases can lead to shock.
In addition to these proinflammatory effects, TNF-α has many other effects directly on the keratinocytes. In fact it facilitates UVB-induced apoptosis and probably contributes to remove damaged cells. This function act sinergically with the chemotactic and leukocyte-activating effect of TNF-α to improve the elimination of dead cells.
Surprisingly recent studies have demonstrated that TNF-α is necessary for the early stages of skin carcinogenesys and development of squamous cells carcinoma. In fact TNF dependent akt/pkb -pkc activation abolishes G2/M checkpoint and diminishes the DNA repair despite induction of apoptosis.
The PKC-Akt axis is likely to be responsible for the TNF-α-induced decrease in DNA repair since sperimental blocking of Akt activity restored DNA repair (16).
The cospique activation of the akt-signaling pathway is mediated by a citoplasmatic protein named ATM that is activated by direct interaction with reactive oxygen species and is able to induce (via the MAP-kinases way) the activity of another protein, TSC2, that in turn inactivate the regulatory protein m-TOR, the principal phosphatase responsible to the inactivation of Akt (17). Akt is involved in many cellular mechanism such as cell growth and proliferation.
Macroscopically, one of the most appreciable sign of solar exposition is actinic hyperkeratosis, that results by the action of TNF-α directly on epidermal cells.
This leads to an increased thickness of stratum corneum and consequently to an improving filter-effect.
This can be considered an adaptative modification of the biologic system, in fact now it is known that the stratum corneum has an important role in scattering solar light (18) (19), decreasing it absolute intensity.
As previously said one of the main effects (direct or not) of UV rays on skin cells is the spontaneous formation of lipid intermediates (peroxydated lipid chains) that have the "role" to inform the cell about the presence of potentially harmful radiations.
These compound, if not removed, potentially drive the cell to an inappropriate response.
The akt/pkb signal pathway also stimulates the autophagy of cell membranes, that lead to an elimination of these compound preventing their prolonged, potentially harmful, effects (20) .
EFFECTS ON FIBROBLASTS
Fibroblast activation by TNF-α and by others interleukines (the most important of these is IL-1 ) leads initially to a migration of dermal fibroblasts to the irradiated epidermis (chemotactic effect), and then to an increased production and liberation of metalloprotease (MMP)-1/interstitial collagenase and MMP-3 (12). The effect of these proteases leads to a degradation of part of the ECM and the basal lamina until generation of characteristic blisters (flittene) of sun scalded skin. The chronicle action of UV on human skin produces a simile-aging (1) effect; in fact several alterations of skin connective tissue that occur during aging are mediated by mechanisms that are similar to those that occur in response to UV irradiation (21).
Thus, skin aging is associated with increased AP-1 activity increased MMP expression , enhanced collagen degradation, and decreased collagen synthesis with consequent ECM degradation that contributes to premature skin aging (photoaging) statistically correlated to sun exposition.
EFFECTS ON MELANOCYTES
Melanocytes are melanin-producing cells located in the stratum basale of epidermis (they are also present in other sites like the middle layer of the uvea, in inner ear, in meninges, in bones and hearth).
These cells are originated from neural crests and, like other neuroepitelial-derived cells, have a elevated activity in oxidation of cyclic aminoacides.
Everyone knows that a delayed consequence of solar exposition of the skin is hyperpigmentation. Melanocytes are the cells responsible for this phenomenon via the production, in specific organelless called melanosomes, of the photopigment melanin.
Melanins are biological polymers derived by aminoacid tyrosine, present virtually in all superior animals.
The first step of melanine synthesis requires tyrosinase, a copper containing protein that oxidates tyrosine to DOPA and subsequently DOPA to DOPA-quinone. Subsequent reactions are mainly spontaneous and lead, finally, to the formation of two distinct families of compound: eumelanins and pheomelanins.
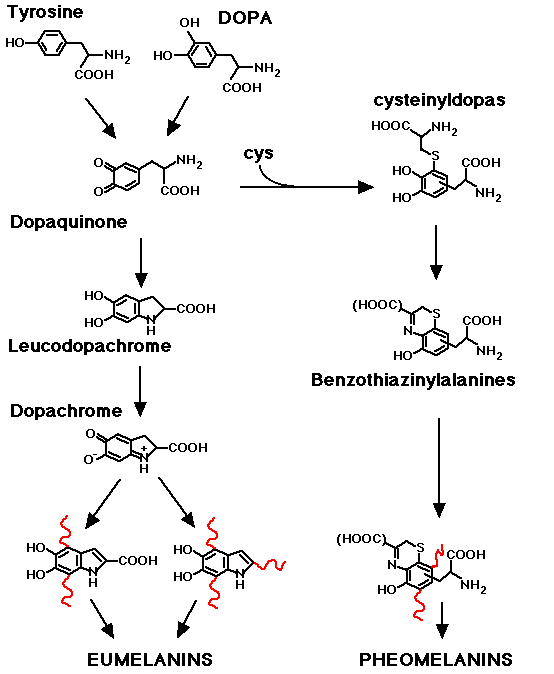
Melanocytes biosynthetic activity is dependent on blood tyrosine level (tyrosinemia). For this reason in PKU patients have a fair skin (22).
The physiological response of melanocyte to sunlight consist in a strong induction of the melanogenesys and in the movement of melanosomes through the cellular dendrites to reach neighbor keratinocytes (in man approximatively 35 KC for each MC).
The activation of melanocytes by sunlight may involve the transformation from a physical signal (UV) to a chemical mediator. Probably this process is carried out by the interaction between a tryptophan photoproduct that generates in cell exposed to UV rays, 6-formylindolo[3,2-b]carbazole (FICZ), and the aryl hydrocarbon receptor (AHR), a citoplasmatic receptor whose ligand was completely unknown until few years ago. This interaction activates the AHR and lead to a complex migration into the nucleus and to the binding to specifics DNA binding sites, modulating several gene transcription (23).

Other studies supposed that also direct DNA damage should induce the melanin synthesis (24).
AHR mediated gene induction increased also the transcription of CYP1A1 gene and this can explain the circadian variability on drugs metabolism (23).
Light activated melanocytes transfer melanin to keratinocytes - mainly to the basal cells - by a pinocytotic process, that is strongly induced by both α-MSH , ACTH via MC-1R receptor and by an increased intracellular c-AMP concentration.
In keratinocytes melanin is still enclosed into melanosomes, that form a cap on the cell nucleus.
Upon exposure to UV light radiation, melanin pigments revealed two distinct photobiological reactions: photoprotective and phototoxic reactions. The intermediate precursor of brown-black eumelanin, 5-6-dihydroxyindole, appears to possess the most potent photoprotective (antioxidant) property. Eumelanin pigment had also some antioxidant properties (25). These pigments induce, in some cases, the (paradoxical) generation of reactive species, and these phototoxyc effect of melanines could be enhanced by free iron, that is also increased in cells exposed to UV (as previously said).
Thus melanin has a potential pro-damaging role in UV exposed skin, and melanocytes front this eventuality segregating all steps of its biosynthesis into membrane-surrounded organelles.
Despite these melanines demonstrate to have an overall potent cell-protective effect in addition to the classical hypothesis of direct UV schermation, it neutralizes radical species (ROS, RNS) produced in solar tissue exposition (26).
Melanosomes are related to the organelles of the endosomal/lysosomal pathway and can have a low internal pH. Is now clear that intraluminal pH is fundamental for melanogenesys: a neutralization of melanosomal pH strongly decreases melanogenesys in human melanocytes (27).
Melanocytes are cells exposed to a very high level of radiations that are powerful mutagenic agents, and because of the embryological origin they are cells prone to migration.
This must be potentially deleterious for the organism because of their facilitating action on the genesis of malignant cancer (melanoma (28) is in fact one of most aggressive cancers in humans).
5,6-dihydroxyindole-2-carboxylic acid (DHICA) is a moiety of the melanine synthesis pathway; it diffuses in a paracrine way to near cells (keratinocytes and melanocytes) and exert a variety of biological roles: it reduces the rate of cell proliferation, enhances the expression of differentiation markers on cell surface and induces the synthesis of antioxidant enzymes.
It can be considered a mechanism to contrast pro-cancerogenic effects of sunlight on skin (29).
This can explain the more dangerous effect rescontrated in sporadic and prolonged sunlight exposition compared to the constant exposition under the sun.
Others sunlight effects:
- Synthesis of vitamin D3 (30);
- Treatment of depression (31);